Regulation of Kif15 localization and motility by the C-terminus of TPX2 and microtubule dynamics
- PMID: 27852894
- PMCID: PMC5221630
- DOI: 10.1091/mbc.E16-06-0476
Regulation of Kif15 localization and motility by the C-terminus of TPX2 and microtubule dynamics
Abstract
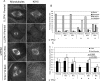
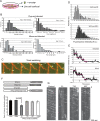
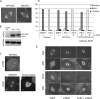
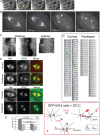
Mitotic motor proteins generate force to establish and maintain spindle bipolarity, but how they are temporally and spatially regulated in vivo is unclear. Prior work demonstrated that a microtubule-associated protein, TPX2, targets kinesin-5 and kinesin-12 motors to spindle microtubules. The C-terminal domain of TPX2 contributes to the localization and motility of the kinesin-5, Eg5, but it is not known whether this domain regulates kinesin-12, Kif15. We found that the C-terminal domain of TPX2 contributes to the localization of Kif15 to spindle microtubules in cells and suppresses motor walking in vitro. Kif15 and Eg5 are partially redundant motors, and overexpressed Kif15 can drive spindle formation in the absence of Eg5 activity. Kif15-dependent bipolar spindle formation in vivo requires the C-terminal domain of TPX2. In the spindle, fluorescent puncta of GFP-Kif15 move toward the equatorial region at a rate equivalent to microtubule growth. Reduction of microtubule growth with paclitaxel suppresses GFP-Kif15 motility, demonstrating that dynamic microtubules contribute to Kif15 behavior. Our results show that the C-terminal region of TPX2 regulates Kif15 in vitro, contributes to motor localization in cells, and is required for Kif15 force generation in vivo and further reveal that dynamic microtubules contribute to Kif15 behavior in vivo.
© 2017 Mann, Balchand, and Wadsworth. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
Similar articles
-
Kif15 cooperates with eg5 to promote bipolar spindle assembly.Curr Biol. 2009 Nov 3;19(20):1703-11. doi: 10.1016/j.cub.2009.08.027. Epub 2009 Oct 8. Curr Biol. 2009. PMID: 19818618
-
Dynamic reorganization of Eg5 in the mammalian spindle throughout mitosis requires dynein and TPX2.Mol Biol Cell. 2012 Apr;23(7):1254-66. doi: 10.1091/mbc.E11-09-0820. Epub 2012 Feb 15. Mol Biol Cell. 2012. PMID: 22337772 Free PMC article.
-
Poleward transport of TPX2 in the mammalian mitotic spindle requires dynein, Eg5, and microtubule flux.Mol Biol Cell. 2010 Mar 15;21(6):979-88. doi: 10.1091/mbc.e09-07-0601. Epub 2010 Jan 28. Mol Biol Cell. 2010. PMID: 20110350 Free PMC article.
-
Chromokinesins: localization-dependent functions and regulation during cell division.Biochem Soc Trans. 2011 Oct;39(5):1154-60. doi: 10.1042/BST0391154. Biochem Soc Trans. 2011. PMID: 21936781 Review.
-
Chromokinesins.Curr Biol. 2018 Oct 8;28(19):R1131-R1135. doi: 10.1016/j.cub.2018.07.017. Curr Biol. 2018. PMID: 30300593 Free PMC article. Review.
Cited by
-
Phragmoplast Orienting Kinesin 2 Is a Weak Motor Switching between Processive and Diffusive Modes.Biophys J. 2018 Jul 17;115(2):375-385. doi: 10.1016/j.bpj.2018.06.012. Biophys J. 2018. PMID: 30021112 Free PMC article.
-
Acentrosomal spindle assembly and maintenance in Caenorhabditis elegans oocytes requires a kinesin-12 nonmotor microtubule interaction domain.Mol Biol Cell. 2022 Jul 1;33(8):ar71. doi: 10.1091/mbc.E22-05-0153. Epub 2022 May 20. Mol Biol Cell. 2022. PMID: 35594182 Free PMC article.
-
Dual localized kinesin-12 POK2 plays multiple roles during cell division and interacts with MAP65-3.EMBO Rep. 2018 Sep;19(9):e46085. doi: 10.15252/embr.201846085. Epub 2018 Jul 12. EMBO Rep. 2018. PMID: 30002118 Free PMC article.
-
The nonmotor adaptor HMMR dampens Eg5-mediated forces to preserve the kinetics and integrity of chromosome segregation.Mol Biol Cell. 2018 Apr 1;29(7):786-796. doi: 10.1091/mbc.E17-08-0531. Mol Biol Cell. 2018. PMID: 29386294 Free PMC article.
-
KIF15 facilitates gastric cancer via enhancing proliferation, inhibiting apoptosis, and predict poor prognosis.Cancer Cell Int. 2020 Apr 15;20:125. doi: 10.1186/s12935-020-01199-7. eCollection 2020. Cancer Cell Int. 2020. PMID: 32322172 Free PMC article.
References
-
- Blangy A, Arnaud L, Nigg EA. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin related motor HsEg5 to the dynactin subunit p150glued. J Biol Chem. 1997;272:19418–19424. - PubMed
-
- Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

