Ras and Rab interactor 1 controls neuronal plasticity by coordinating dendritic filopodial motility and AMPA receptor turnover
- PMID: 27852895
- PMCID: PMC5231897
- DOI: 10.1091/mbc.E16-07-0526
Ras and Rab interactor 1 controls neuronal plasticity by coordinating dendritic filopodial motility and AMPA receptor turnover
Abstract
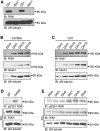
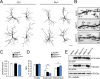
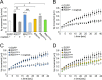
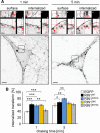
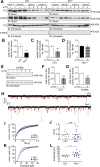
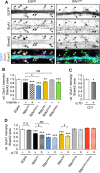
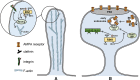
Ras and Rab interactor 1 (RIN1) is predominantly expressed in the nervous system. RIN1-knockout animals have deficits in latent inhibition and fear extinction in the amygdala, suggesting a critical role for RIN1 in preventing the persistence of unpleasant memories. At the molecular level, RIN1 signals through Rab5 GTPases that control endocytosis of cell-surface receptors and Abl nonreceptor tyrosine kinases that participate in actin cytoskeleton remodeling. Here we report that RIN1 controls the plasticity of cultured mouse hippocampal neurons. Our results show that RIN1 affects the morphology of dendritic protrusions and accelerates dendritic filopodial motility through an Abl kinase-dependent pathway. Lack of RIN1 results in enhanced mEPSC amplitudes, indicating an increase in surface AMPA receptor levels compared with wild-type neurons. We further provide evidence that the Rab5 GEF activity of RIN1 regulates surface GluA1 subunit endocytosis. Consequently loss of RIN1 blocks surface AMPA receptor down-regulation evoked by chemically induced long-term depression. Our findings indicate that RIN1 destabilizes synaptic connections and is a key player in postsynaptic AMPA receptor endocytosis, providing multiple ways of negatively regulating memory stabilization during neuronal plasticity.
© 2017 Szíber et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
References
-
- Arendt T, Gärtner U, Seeger G, Barmashenko G, Palm K, Mittmann T, Yan L, Hümmeke M, Behrbohm J, Brückner MK, et al. Neuronal activation of Ras regulates synaptic connectivity. Eur J Neurosci. 2004;19:2953–2966. - PubMed
-
- Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry. 2006;60:322–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

