Reconstituting regulation of the canonical Wnt pathway by engineering a minimal β-catenin destruction machine
- PMID: 27852897
- PMCID: PMC5221518
- DOI: 10.1091/mbc.E16-07-0557
Reconstituting regulation of the canonical Wnt pathway by engineering a minimal β-catenin destruction machine
Abstract
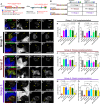
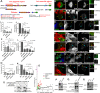
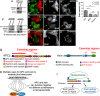
Negatively regulating key signaling pathways is critical to development and altered in cancer. Wnt signaling is kept off by the destruction complex, which is assembled around the tumor suppressors APC and Axin and targets β-catenin for destruction. Axin and APC are large proteins with many domains and motifs that bind other partners. We hypothesized that if we identified the essential regions required for APC:Axin cooperative function and used these data to design a minimal β-catenin-destruction machine, we would gain new insights into the core mechanisms of destruction complex function. We identified five key domains/motifs in APC or Axin that are essential for their function in reconstituting Wnt regulation. Strikingly, however, certain APC and Axin mutants that are nonfunctional on their own can complement one another in reducing β-catenin, revealing that the APC:Axin complex is a highly robust machine. We used these insights to design a minimal β-catenin-destruction machine, revealing that a minimized chimeric protein covalently linking the five essential regions of APC and Axin reconstitutes destruction complex internal structure, size, and dynamics, restoring efficient β-catenin destruction in colorectal tumor cells. On the basis of our data, we propose a new model of the mechanistic function of the destruction complex as an integrated machine.
© 2017 Pronobis et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




Similar articles
-
Wnt/Beta-Catenin Signaling Regulation and a Role for Biomolecular Condensates.Dev Cell. 2019 Feb 25;48(4):429-444. doi: 10.1016/j.devcel.2019.01.025. Dev Cell. 2019. PMID: 30782412 Free PMC article. Review.
-
A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient βcatenin destruction.Elife. 2015 Sep 22;4:e08022. doi: 10.7554/eLife.08022. Elife. 2015. PMID: 26393419 Free PMC article.
-
The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway.PLoS Biol. 2003 Oct;1(1):E10. doi: 10.1371/journal.pbio.0000010. Epub 2003 Oct 13. PLoS Biol. 2003. PMID: 14551908 Free PMC article.
-
Identification of ICAT as an APC Inhibitor, Revealing Wnt-Dependent Inhibition of APC-Axin Interaction.Mol Cell. 2018 Oct 4;72(1):37-47.e4. doi: 10.1016/j.molcel.2018.07.040. Epub 2018 Sep 6. Mol Cell. 2018. PMID: 30197296
-
[Dual-role regulations of canonical Wnt/beta-catenin signaling pathway].Beijing Da Xue Xue Bao Yi Xue Ban. 2010 Apr 18;42(2):238-42. Beijing Da Xue Xue Bao Yi Xue Ban. 2010. PMID: 20396373 Review. Chinese.
Cited by
-
Oncogenic Mutations in Armadillo Repeats 5 and 6 of β-Catenin Reduce Binding to APC, Increasing Signaling and Transcription of Target Genes.Gastroenterology. 2020 Mar;158(4):1029-1043.e10. doi: 10.1053/j.gastro.2019.11.302. Epub 2019 Dec 17. Gastroenterology. 2020. PMID: 31857074 Free PMC article.
-
Nucleation of the destruction complex on the centrosome accelerates degradation of β-catenin and regulates Wnt signal transmission.Proc Natl Acad Sci U S A. 2022 Sep 6;119(36):e2204688119. doi: 10.1073/pnas.2204688119. Epub 2022 Aug 29. Proc Natl Acad Sci U S A. 2022. PMID: 36037369 Free PMC article.
-
Wnt regulation: exploring Axin-Disheveled interactions and defining mechanisms by which the SCF E3 ubiquitin ligase is recruited to the destruction complex.Mol Biol Cell. 2020 May 1;31(10):992-1014. doi: 10.1091/mbc.E19-11-0647. Epub 2020 Mar 4. Mol Biol Cell. 2020. PMID: 32129710 Free PMC article.
-
Wnt/Beta-Catenin Signaling Regulation and a Role for Biomolecular Condensates.Dev Cell. 2019 Feb 25;48(4):429-444. doi: 10.1016/j.devcel.2019.01.025. Dev Cell. 2019. PMID: 30782412 Free PMC article. Review.
-
Mechanisms of Wnt signaling and control.Wiley Interdiscip Rev Syst Biol Med. 2018 Sep;10(5):e1422. doi: 10.1002/wsbm.1422. Epub 2018 Mar 30. Wiley Interdiscip Rev Syst Biol Med. 2018. PMID: 29600540 Free PMC article. Review.
References
-
- Ahmed Y, Nouri A, Wieschaus E. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development. 2002;129:1751–1762. - PubMed
-
- Akong K, Grevengoed E, Price M, McCartney B, Hayden M, DeNofrio J, Peifer M. Drosophila APC2 and APC1 play overlapping roles in wingless signaling in the embryo and imaginal discs. Dev Biol. 2002;250:91–100. - PubMed
-
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

