Phosphorylation induces distinct alpha-synuclein strain formation
- PMID: 27853185
- PMCID: PMC5112567
- DOI: 10.1038/srep37130
Phosphorylation induces distinct alpha-synuclein strain formation
Abstract
Synucleinopathies are a group of neurodegenerative diseases associated with alpha-synuclein (α-Syn) aggregation. Recently, increasing evidence has demonstrated the existence of different structural characteristics or 'strains' of α-Syn, supporting the concept that synucleinopathies share several common features with prion diseases and possibly explaining how a single protein results in different clinical phenotypes within synucleinopathies. In earlier studies, the different strains were generated through the regulation of solution conditions, temperature, or repetitive seeded fibrillization in vitro. Here, we synthesize homogeneous α-Syn phosphorylated at serine 129 (pS129 α-Syn), which is highly associated with the pathological changes, and demonstrate that phosphorylation at Ser129 induces α-Syn to form a distinct strain with different structures, propagation properties, and higher cytotoxicity compared with the wild-type α-Syn. The results are the first demonstration that post-translational modification of α-Syn can induce different strain formation, offering a new mechanism for strain formation.
Figures

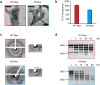
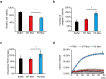
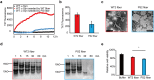
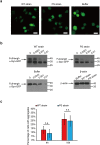
References
-
- Goedert M., Clavaguera F. & Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 33, 317–325 (2010). - PubMed
-
- Li J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

