Modulation of light-driven arousal by LIM-homeodomain transcription factor Apterous in large PDF-positive lateral neurons of the Drosophila brain
- PMID: 27853240
- PMCID: PMC5112534
- DOI: 10.1038/srep37255
Modulation of light-driven arousal by LIM-homeodomain transcription factor Apterous in large PDF-positive lateral neurons of the Drosophila brain
Abstract
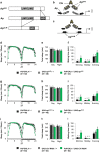
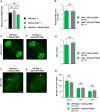
Apterous (Ap), the best studied LIM-homeodomain transcription factor in Drosophila, cooperates with the cofactor Chip (Chi) to regulate transcription of specific target genes. Although Ap regulates various developmental processes, its function in the adult brain remains unclear. Here, we report that Ap and Chi in the neurons expressing PDF, a neuropeptide, play important roles in proper sleep/wake regulation in adult flies. PDF-expressing neurons consist of two neuronal clusters: small ventral-lateral neurons (s-LNvs) acting as the circadian pacemaker and large ventral-lateral neurons (l-LNvs) regulating light-driven arousal. We identified that Ap localizes to the nuclei of s-LNvs and l-LNvs. In light-dark (LD) cycles, RNAi knockdown or the targeted expression of dominant-negative forms of Ap or Chi in PDF-expressing neurons or l-LNvs promoted arousal. In contrast, in constant darkness, knockdown of Ap in PDF-expressing neurons did not promote arousal, indicating that a reduced Ap function in PDF-expressing neurons promotes light-driven arousal. Furthermore, Ap expression in l-LNvs showed daily rhythms (peaking at midnight), which are generated by a direct light-dependent mechanism rather than by the endogenous clock. These results raise the possibility that the daily oscillation of Ap expression in l-LNvs may contribute to the buffering of light-driven arousal in wild-type flies.
Figures





References
-
- Hobert O. & Westphal H. Functions of LIM-homeobox genes. Trends Genet. 16, 75–83 (2000). - PubMed
-
- Cohen B., McGuffin M. E., Pfeifle C., Segal D. & Cohen S. M. apterous, a gene required for imaginal disc development in Drosophila encodes a member of the LIM family of developmental regulatory proteins. Genes Dev. 6, 715–729 (1992). - PubMed
-
- O′Keefe D. D., Thor S. & Thomas J. B. Function and specificity of LIM domains in Drosophila nervous system and wing development. Development 125, 3915–3923 (1998). - PubMed
-
- Bourgouin C., Lundgren S. E. & Thomas J. B. Apterous is a Drosophila LIM domain gene required for the development of a subset of embryonic muscles. Neuron 9, 549–561 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

