Lent-On-Plus Lentiviral vectors for conditional expression in human stem cells
- PMID: 27853296
- PMCID: PMC5112523
- DOI: 10.1038/srep37289
Lent-On-Plus Lentiviral vectors for conditional expression in human stem cells
Abstract
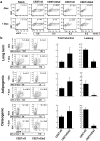
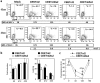
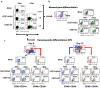
Conditional transgene expression in human stem cells has been difficult to achieve due to the low efficiency of existing delivery methods, the strong silencing of the transgenes and the toxicity of the regulators. Most of the existing technologies are based on stem cells clones expressing appropriate levels of tTA or rtTA transactivators (based on the TetR-VP16 chimeras). In the present study, we aim the generation of Tet-On all-in-one lentiviral vectors (LVs) that tightly regulate transgene expression in human stem cells using the original TetR repressor. By using appropriate promoter combinations and shielding the LVs with the Is2 insulator, we have constructed the Lent-On-Plus Tet-On system that achieved efficient transgene regulation in human multipotent and pluripotent stem cells. The generation of inducible stem cell lines with the Lent-ON-Plus LVs did not require selection or cloning, and transgene regulation was maintained after long-term cultured and upon differentiation toward different lineages. To our knowledge, Lent-On-Plus is the first all-in-one vector system that tightly regulates transgene expression in bulk populations of human pluripotent stem cells and its progeny.
Conflict of interest statement
The authors have applied for an spanish patent “Lent-On-Plus system for conditional expression on human stem cells”. Spanish application number: P201631406 Applicant: Fundación Progreso y Salud, Date of receipt 4 November 2016. This patent application does not alter the authors’ adherence to all the Scientific Report journal policies on sharing data and materials. Therefore all materials presented in the manuscript will be freely available to the scientific community although not for commercial purposes.
Figures




References
-
- Ting D. T., Kyba M. & Daley G. Q. Inducible transgene expression in mouse stem cells. Methods Mol Med 105, 23–46 (2005). - PubMed
-
- Vieyra D. S. & Goodell M. A. Pluripotentiality and conditional transgene regulation in human embryonic stem cells expressing insulated tetracycline-ON transactivator. Stem Cells 25, 2559–2566 (2007). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

