Human blood myeloid and plasmacytoid dendritic cells cross activate each other and synergize in inducing NK cell cytotoxicity
- PMID: 27853652
- PMCID: PMC5087293
- DOI: 10.1080/2162402X.2016.1227902
Human blood myeloid and plasmacytoid dendritic cells cross activate each other and synergize in inducing NK cell cytotoxicity
Abstract
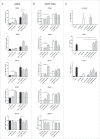
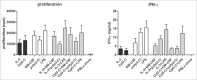
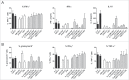
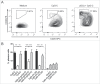
Human blood dendritic cells (DCs) hold great potential for use in anticancer immunotherapies. CD1c+ myeloid DCs and plasmacytoid DCs (pDCs) have been successfully utilized in clinical vaccination trials against melanoma. We hypothesize that combining both DC subsets in a single vaccine can further improve vaccine efficacy. Here, we have determined the potential synergy between the two subsets in vitro on the level of maturation, cytokine expression, and effector cell induction. Toll-like receptor (TLR) stimulation of CD1c+ DCs induced cross-activation of immature pDCs and vice versa. When both subsets were stimulated together using TLR agonists, CD86 expression on pDCs was increased and higher levels of interferon (IFN)-α were produced by DC co-cultures. Although the two subsets did not display any synergistic effect on naive CD4+ and CD8+ T cell polarization, CD1c+ DCs and pDCs were able to complement each other's induction of other immune effector cells. The mere presence of pDCs in DC co-cultures promoted plasma cell differentiation from activated autologous B cells. Similarly, CD1c+ DCs, alone or in co-cultures, induced high levels of IFN-γ from allogeneic peripheral blood lymphocytes or activated autologous natural killer (NK) cells. Both CD1c+ DCs and pDCs could enhance NK cell cytotoxicity, and interestingly DC co-cultures further enhanced NK cell-mediated killing of an NK-resistant tumor cell line. These results indicate that co-application of human blood DC subsets could render DC-based anticancer vaccines more efficacious.
Keywords: Adaptive immunity; NK cells; cancer immunotherapy; crosstalk; myeloid DCs; plasmacytoid DCs.
Figures
Similar articles
-
Human CD34+-derived complete plasmacytoid and conventional dendritic cell vaccine effectively induces antigen-specific CD8+ T cell and NK cell responses in vitro and in vivo.Cell Mol Life Sci. 2023 Sep 20;80(10):298. doi: 10.1007/s00018-023-04923-4. Cell Mol Life Sci. 2023. PMID: 37728691 Free PMC article.
-
Naturally produced type I IFNs enhance human myeloid dendritic cell maturation and IL-12p70 production and mediate elevated effector functions in innate and adaptive immune cells.Cancer Immunol Immunother. 2018 Sep;67(9):1425-1436. doi: 10.1007/s00262-018-2204-2. Epub 2018 Jul 13. Cancer Immunol Immunother. 2018. PMID: 30019146 Free PMC article.
-
Phenotypic and Transcriptomic Analysis of Peripheral Blood Plasmacytoid and Conventional Dendritic Cells in Early Drug Naïve Rheumatoid Arthritis.Front Immunol. 2018 May 9;9:755. doi: 10.3389/fimmu.2018.00755. eCollection 2018. Front Immunol. 2018. PMID: 29867920 Free PMC article.
-
Natural killer-dendritic cell cross-talk in cancer immunotherapy.Expert Opin Biol Ther. 2005 Oct;5(10):1303-15. doi: 10.1517/14712598.5.10.1303. Expert Opin Biol Ther. 2005. PMID: 16197336 Review.
-
The clinical application of cancer immunotherapy based on naturally circulating dendritic cells.J Immunother Cancer. 2019 Apr 18;7(1):109. doi: 10.1186/s40425-019-0580-6. J Immunother Cancer. 2019. PMID: 30999964 Free PMC article. Review.
Cited by
-
The Multifaceted Functionality of Plasmacytoid Dendritic Cells in Gastrointestinal Cancers: A Potential Therapeutic Target?Cancers (Basel). 2024 Jun 13;16(12):2216. doi: 10.3390/cancers16122216. Cancers (Basel). 2024. PMID: 38927922 Free PMC article. Review.
-
Characterization and Manipulation of the Crosstalk Between Dendritic and Natural Killer Cells Within the Tumor Microenvironment.Front Immunol. 2021 May 14;12:670540. doi: 10.3389/fimmu.2021.670540. eCollection 2021. Front Immunol. 2021. PMID: 34054844 Free PMC article. Review.
-
Application of Engineered Dendritic Cell Vaccines in Cancer Immunotherapy: Challenges and Opportunities.Curr Treat Options Oncol. 2023 Dec;24(12):1703-1719. doi: 10.1007/s11864-023-01143-7. Epub 2023 Nov 14. Curr Treat Options Oncol. 2023. PMID: 37962824 Review.
-
Roles of Plasmacytoid Dendritic Cells in Gastric Cancer.Front Oncol. 2022 Mar 3;12:818314. doi: 10.3389/fonc.2022.818314. eCollection 2022. Front Oncol. 2022. PMID: 35311157 Free PMC article. Review.
-
Interleukin-15-Cultured Dendritic Cells Enhance Anti-Tumor Gamma Delta T Cell Functions through IL-15 Secretion.Front Immunol. 2018 Apr 10;9:658. doi: 10.3389/fimmu.2018.00658. eCollection 2018. Front Immunol. 2018. PMID: 29692776 Free PMC article.
References
-
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; http://dx.doi.org/10.1038/nrc3258 - DOI - PMC - PubMed
-
- Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 2014; 15:e257-67; PMID:24872109; http://dx.doi.org/10.1016/S1470-2045(13)70585-0 - DOI - PubMed
-
- Wimmers F, Schreibelt G, Sköld AE, Figdor CG, De Vries IJM. Paradigm shift in dendritic cell-based immunotherapy: from in vitro generated monocyte-derived DCs to naturally circulating DC subsets. Front Immunol 2014; 5:165; PMID:24782868; http://dx.doi.org/10.3389/fimmu.2014.00165 - DOI - PMC - PubMed
-
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol 2000; 165:6037-46; PMID:11086035; http://dx.doi.org/10.4049/jimmunol.165.11.6037 - DOI - PubMed
-
- Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol 2001; 31:3388-93; PMID:11745357; http://dx.doi.org/10.1002/1521-4141(200111)31:11%3c3388::AID-IMMU3388%3e... - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
