Longitudinal assessment of pain management with the pain management index in cancer outpatients receiving chemotherapy
- PMID: 27853929
- PMCID: PMC5266766
- DOI: 10.1007/s00520-016-3482-x
Longitudinal assessment of pain management with the pain management index in cancer outpatients receiving chemotherapy
Abstract
Purpose: The adequacy of pain management for individuals with cancer who receive outpatient chemotherapy is unclear. The primary objective of this study was to assess pain prevalence and intensity in such patients. The secondary objectives included assessment of pain management with the pain management index (PMI) and exploration of predictors of inadequate pain management.
Methods: Cancer patients who received outpatient chemotherapy were enrolled. Patients were required to complete questionnaires covering demographic data and including the Brief Pain Inventory and the Distress Thermometer and Impact Thermometer. The PMI score was determined twice with an interval of at least 3 weeks.
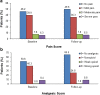
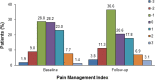
Results: Of the 740 patients enrolled in the study, 524 individuals (70.8%) completed all questionnaires. Totals of 282 patients (53.8%) and 264 patients (50.4%) reported pain at baseline and follow-up, respectively, with ∼14% of patients having moderate or severe pain at each assessment. Totals of 365 patients (69.7%) at baseline and 320 patients (61.1%) at follow-up reported pain or were prescribed analgesics, with the rate of inadequate pain management for these patients being 39.7 and 51.6%, respectively. Multivariable analysis for 418 patients (79.8%) who had pain or required analgesics at baseline or follow-up (or both) revealed that the most significant predictor of inadequate pain management was depressive state.
Conclusions: Pain in cancer patients receiving outpatient chemotherapy is prevalent and at risk for undertreatment. Pain management should be assessed on a regular basis and is likely to be improved by screening for depression.
Keywords: Cancer; Depressive state; Inadequate pain management; Outpatient chemotherapy; Pain; Pain management index (PMI).
Conflict of interest statement
Compliance with ethical standards Conflicts of interest K.T. and Y.N. have received educational grants from Janssen Pharmaceutical K.K. All remaining authors have declared no conflicts of interest. Ethical approval The study design was approved by the institutional ethics review board of Kyushu University Hospital and complied with the 1964 Helsinki Declaration and its later amendments. Informed consent Written informed consent was obtained from all individual participants included in the study when they visited the OCU for orientation before starting outpatient chemotherapy.
Figures

References
-
- Fisch MJ, Lee JW, Weiss M, Wagner LI, Chang VT, Cella D, Manola JB, Minasian LM, McCaskill-Stevens W, Mendoza TR, Cleeland CS. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol. 2012;30:1980–1988. doi: 10.1200/JCO.2011.39.2381. - DOI - PMC - PubMed
-
- von Moos R, Body JJ, Egerdie B, Stopeck A, Brown J, Fallowfield L, Patrick DL, Cleeland C, Damyanov D, Palazzo FS, Marx G, Zhou Y, Braun A, Balakumaran A, Qian Y. Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support Care Cancer. 2016;24:1327–1337. doi: 10.1007/s00520-015-2908-1. - DOI - PMC - PubMed
-
- Dollinger M. Guidelines for hospitalization for chemotherapy. Oncologist. 1996;1:107–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

