Clinical outcomes and case volume effect of transanal total mesorectal excision for rectal cancer: a systematic review
- PMID: 27853973
- PMCID: PMC5156667
- DOI: 10.1007/s10151-016-1545-0
Clinical outcomes and case volume effect of transanal total mesorectal excision for rectal cancer: a systematic review
Abstract
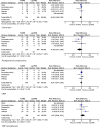
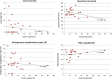
Transanal total mesorectal excision (TaTME) has been developed to improve quality of TME for patients with mid and low rectal cancer. However, despite enthusiastic uptake and teaching facilities, concern exists for safe introduction. TaTME is a complex procedure and potentially a learning curve will hamper clinical outcome. With this systematic review, we aim to provide data regarding morbidity and safety of TaTME. A systematic literature search was performed in MEDLINE (PubMed), EMBASE (Ovid) and Cochrane Library. Case reports, cohort series and comparative series on TaTME for rectal cancer were included. To evaluate a potential effect of case volume, low-volume centres (n ≤ 30 total volume) were compared with high-volume centres (n > 30 total volume). Thirty-three studies were identified (three case reports, 25 case series, five comparative studies), including 794 patients. Conversion was performed in 3.0% of the procedures. The complication rate was 40.3, and 11.5% were major complications. The quality of the mesorectum was "complete" in 87.6%, and the circumferential resection margin (CRM) was involved in 4.7%. In low- versus high-volume centres, the conversion rate was 4.3 versus 2.7%, and major complication rates were 12.2 versus 10.5%, respectively. TME quality was "complete" in 80.5 versus 89.7%, and CRM involvement was 4.8 and 4.5% in low- versus high-volume centres, respectively. TaTME for mid and low rectal cancer is a promising technique; however, it is associated with considerable morbidity. Safe implementation of the TaTME should include proctoring and quality assurance preferably within a trial setting.
Keywords: Case volume; Morbidity; Rectal cancer; Total mesorectal excision; Transanal.
Conflict of interest statement
The authors declare that they have no conflict of interest. Ethical approval This article does not contain any studies with human participants performed by any of the authors. Informed consent For this type of study formal consent is not required.
Figures
References
-
- Guillou PJ, Quirke P, Thorpe H, MRC CLASICC Trial Group et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. - DOI - PubMed
-
- Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. - DOI - PMC - PubMed
-
- van der Pas MH, Haglind E, Cuesta MA, Colorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group et al (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources