Visualization of Oxidative Stress Induced by Experimental Periodontitis in Keap1-Dependent Oxidative Stress Detector-Luciferase Mice
- PMID: 27854327
- PMCID: PMC5133905
- DOI: 10.3390/ijms17111907
Visualization of Oxidative Stress Induced by Experimental Periodontitis in Keap1-Dependent Oxidative Stress Detector-Luciferase Mice
Abstract
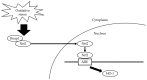
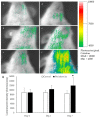
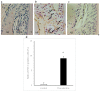
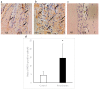
The aim of this study was to investigate whether a Keap1-dependent oxidative stress detector-luciferase (OKD-LUC) mouse model would be useful for the visualization of oxidative stress induced by experimental periodontitis. A ligature was placed around the mandibular first molars for seven days to induce periodontitis. Luciferase activity was measured with an intraperitoneal injection of d-luciferin on days 0, 1, and 7. The luciferase activity in the periodontitis group was significantly greater than that in the control group at seven days. The expressions of heme oxygenase-1 (HO-1) and malondialdehyde in periodontal tissue were significantly higher in the periodontitis group than in the control group. Immunofluorescent analysis confirmed that the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) occurred more frequently in the periodontitis group than in the control group. This study found that under oxidative stress induced by experimental periodontitis, the Nrf2/antioxidant defense pathway was activated and could be visualized from the luciferase activity in the OKD-LUC model. Thus, the OKD-LUC mouse model may be useful for exploring the mechanism underlying the relationship between the Nrf2/antioxidant defense pathway and periodontitis by enabling the visualization of oxidative stress over time.
Keywords: Nrf2; heme oxygenase-1; luciferase activity; oxidative stress; periodontitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

