Indel variant analysis of short-read sequencing data with Scalpel
- PMID: 27854363
- PMCID: PMC5507611
- DOI: 10.1038/nprot.2016.150
Indel variant analysis of short-read sequencing data with Scalpel
Abstract
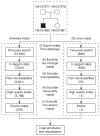
As the second most common type of variation in the human genome, insertions and deletions (indels) have been linked to many diseases, but the discovery of indels of more than a few bases in size from short-read sequencing data remains challenging. Scalpel (http://scalpel.sourceforge.net) is an open-source software for reliable indel detection based on the microassembly technique. It has been successfully used to discover mutations in novel candidate genes for autism, and it is extensively used in other large-scale studies of human diseases. This protocol gives an overview of the algorithm and describes how to use Scalpel to perform highly accurate indel calling from whole-genome and whole-exome sequencing data. We provide detailed instructions for an exemplary family-based de novo study, but we also characterize the other two supported modes of operation: single-sample and somatic analysis. Indel normalization, visualization and annotation of the mutations are also illustrated. Using a standard server, indel discovery and characterization in the exonic regions of the example sequencing data can be completed in ∼5 h after read mapping.
Conflict of interest statement
Figures


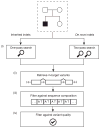
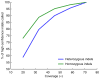
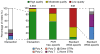
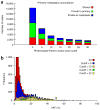



References
-
- Watson JD, Baker TA, Gann A, Levine M, Losick R. Molecular Biology of the Gene. 7. Cold Spring Harbor Laboratory Press; 2013.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

