Characterization of a vraG Mutant in a Genetically Stable Staphylococcus aureus Small-Colony Variant and Preliminary Assessment for Use as a Live-Attenuated Vaccine against Intrammamary Infections
- PMID: 27855187
- PMCID: PMC5113970
- DOI: 10.1371/journal.pone.0166621
Characterization of a vraG Mutant in a Genetically Stable Staphylococcus aureus Small-Colony Variant and Preliminary Assessment for Use as a Live-Attenuated Vaccine against Intrammamary Infections
Abstract
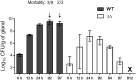
Staphylococcus aureus is a leading cause of bovine intramammary infections (IMIs) that can evolve into difficult-to-treat chronic mastitis. To date, no vaccine formulation has shown high protective efficacy against S. aureus IMI, partly because this bacterium can efficiently evade the immune system. For instance, S. aureus small colony variants (SCVs) have intracellular abilities and can persist without producing invasive infections. As a first step towards the development of a live vaccine, this study describes the elaboration of a novel attenuated mutant of S. aureus taking advantage of the SCV phenotype. A genetically stable SCV was created through the deletion of the hemB gene, impairing its ability to adapt and revert to the invasive phenotype. Further attenuation was obtained through inactivation of gene vraG (SACOL0720) which we previously showed to be important for full virulence during bovine IMIs. After infection of bovine mammary epithelial cells (MAC-T), the double mutant (ΔvraGΔhemB) was less internalized and caused less cell destruction than that seen with ΔhemB and ΔvraG, respectively. In a murine IMI model, the ΔvraGΔhemB mutant was strongly attenuated, with a reduction of viable counts of up to 5-log10 CFU/g of mammary gland when compared to the parental strain. A complete clearance of ΔvraGΔhemB from glands was observed whereas mortality rapidly (48h) occurred with the wild-type strain. Immunization of mice using subcutaneous injections of live ΔvraGΔhemB raised a strong immune response as judged by the high total IgG titers measured against bacterial cell extracts and by the high IgG2a/IgG1 ratio observed against the IsdH protein. Also, ΔvraGΔhemB had sufficient common features with bovine mastitis strains so that the antibody response also strongly recognized strains from a variety of mastitis associated spa types. This double mutant could serve as a live-attenuated component in vaccines to improve cell-mediated immune responses against S. aureus IMIs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

