The Devil Is in the Details: Incomplete Reporting in Preclinical Animal Research
- PMID: 27855228
- PMCID: PMC5113978
- DOI: 10.1371/journal.pone.0166733
The Devil Is in the Details: Incomplete Reporting in Preclinical Animal Research
Abstract
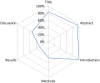
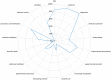
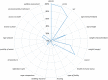
Incomplete reporting of study methods and results has become a focal point for failures in the reproducibility and translation of findings from preclinical research. Here we demonstrate that incomplete reporting of preclinical research is not limited to a few elements of research design, but rather is a broader problem that extends to the reporting of the methods and results. We evaluated 47 preclinical research studies from a systematic review of acute lung injury that use mesenchymal stem cells (MSCs) as a treatment. We operationalized the ARRIVE (Animal Research: Reporting of In Vivo Experiments) reporting guidelines for pre-clinical studies into 109 discrete reporting sub-items and extracted 5,123 data elements. Overall, studies reported less than half (47%) of all sub-items (median 51 items; range 37-64). Across all studies, the Methods Section reported less than half (45%) and the Results Section reported less than a third (29%). There was no association between journal impact factor and completeness of reporting, which suggests that incomplete reporting of preclinical research occurs across all journals regardless of their perceived prestige. Incomplete reporting of methods and results will impede attempts to replicate research findings and maximize the value of preclinical studies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Cressey D. Surge in support for animal-research guidelines. Nature. 2016; 10.1038/nature.2016.19274 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

