Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial
- PMID: 27856208
- PMCID: PMC5291766
- DOI: 10.1016/j.jacc.2016.11.009
Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial
Abstract
Background: Although human mesenchymal stem cells (hMSCs) have been tested in ischemic cardiomyopathy, few studies exist in chronic nonischemic dilated cardiomyopathy (NIDCM).
Objectives: The authors conducted a randomized comparison of safety and efficacy of autologous (auto) versus allogeneic (allo) bone marrow-derived hMSCs in NIDCM.
Methods: Thirty-seven patients were randomized to either allo- or auto-hMSCs in a 1:1 ratio. Patients were recruited between December 2011 and July 2015 at the University of Miami Hospital. Patients received hMSCs (100 million) by transendocardial stem cell injection in 10 left ventricular sites. Treated patients were evaluated at baseline, 30 days, and 3-, 6-, and 12-months for safety (serious adverse events [SAE]), and efficacy endpoints: ejection fraction, Minnesota Living with Heart Failure Questionnaire, 6-min walk test, major adverse cardiac events, and immune biomarkers.
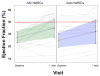
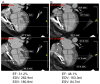
Results: There were no 30-day treatment-emergent SAEs. Twelve-month SAE incidence was 28.2% with allo-hMSCs versus 63.5% with auto-hMSCs (p = 0.1004 for the comparison). One allo-hMSC patient developed an elevated (>80) donor-specific calculated panel reactive antibody level. The ejection fraction increased in allo-hMSC patients by 8.0 percentage points (p = 0.004) compared with 5.4 with auto-hMSCs (p = 0.116; allo vs. auto p = 0.4887). The 6-min walk test increased with allo-hMSCs by 37.0 m (p = 0.04), but not auto-hMSCs at 7.3 m (p = 0.71; auto vs. allo p = 0.0168). MLHFQ score decreased in allo-hMSC (p = 0.0022) and auto-hMSC patients (p = 0.463; auto vs. allo p = 0.172). The major adverse cardiac event rate was lower, too, in the allo group (p = 0.0186 vs. auto). Tumor necrosis factor-α decreased (p = 0.0001 for each), to a greater extent with allo-hMSCs versus auto-hMSCs at 6 months (p = 0.05).
Conclusions: These findings demonstrated safety and clinically meaningful efficacy of allo-hMSC versus auto-hMSC in NIDCM patients. Pivotal trials of allo-hMSCs are warranted based on these results. (Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy [PoseidonDCM]; NCT01392625).
Keywords: endothelial function; heart failure; idiopathic dilated cardiomyopathy; immune biomarker; stem cell therapy.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interest: Dr. Hare and Dr. Heldman disclose a relationship with Vestion Inc that includes equity, board membership, and consulting. Dr. Hatzistergos and K. Valasaki disclose a relationship with Vestion Inc that includes equity. Vestion Inc did not participate in funding this work. Dr. Landin, Dr. Hare, A. Khan, and D. DiFede disclose a relationship with Longeveron LLC that includes consulting. Longeveron LLC did not participate in funding this work. D. DiFede discloses a relationship with BDS as consultant. The other authors report no conflicts.
Figures



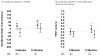
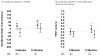
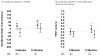
Comment in
-
Mesenchymal Cell Therapy for Dilated Cardiomyopathy: Time to Test the Water.J Am Coll Cardiol. 2017 Feb 7;69(5):538-540. doi: 10.1016/j.jacc.2016.11.044. J Am Coll Cardiol. 2017. PMID: 28153109 No abstract available.
-
Stem cells: Allogenic mesenchymal stem cells for dilated cardiomyopathy.Nat Rev Cardiol. 2017 Apr;14(4):190-191. doi: 10.1038/nrcardio.2017.21. Epub 2017 Feb 16. Nat Rev Cardiol. 2017. PMID: 28202901 No abstract available.
References
-
- Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. - PubMed
-
- Kirklin JK, Cantor R, Mohacsi P, et al. First Annual IMACS Report: A global International Society for Heart and Lung Transplantation Registry for Mechanical Circulatory Support. J Heart Lung Transplant. 2016;35:407–12. - PubMed
-
- Patel AN, Henry TD, Quyyumi AA, et al. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet. 2016;387:2412–21. - PubMed
-
- Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

