Aspirin increases mitochondrial fatty acid oxidation
- PMID: 27856258
- PMCID: PMC5195905
- DOI: 10.1016/j.bbrc.2016.11.066
Aspirin increases mitochondrial fatty acid oxidation
Abstract
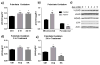
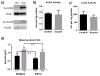
The metabolic effects of salicylates are poorly understood. This study investigated the effects of aspirin on fatty acid oxidation. Aspirin increased mitochondrial long-chain fatty acid oxidation, but inhibited peroxisomal fatty acid oxidation, in two different cell lines. Aspirin increased mitochondrial protein acetylation and was found to be a stronger acetylating agent in vitro than acetyl-CoA. However, aspirin-induced acetylation did not alter the activity of fatty acid oxidation proteins, and knocking out the mitochondrial deacetylase SIRT3 did not affect the induction of long-chain fatty acid oxidation by aspirin. Aspirin did not change oxidation of medium-chain fatty acids, which can freely traverse the mitochondrial membrane. Together, these data indicate that aspirin does not directly alter mitochondrial matrix fatty acid oxidation enzymes, but most likely exerts its effects at the level of long-chain fatty acid transport into mitochondria. The drive on mitochondrial fatty acid oxidation may be a compensatory response to altered mitochondrial morphology and inhibited electron transport chain function, both of which were observed after 24 h incubation of cells with aspirin. These studies provide insight into the pathophysiology of Reye Syndrome, which is known to be triggered by aspirin ingestion in patients with fatty acid oxidation disorders.
Keywords: Aspirin; Fatty acid oxidation; Lysine acetylation; Mitochondria; Peroxisomes; SIRT3.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

