Neurotrophic and neuroprotective effects of oxyntomodulin in neuronal cells and a rat model of stroke
- PMID: 27856285
- PMCID: PMC5203805
- DOI: 10.1016/j.expneurol.2016.11.010
Neurotrophic and neuroprotective effects of oxyntomodulin in neuronal cells and a rat model of stroke
Abstract
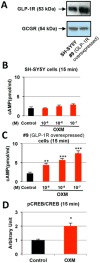
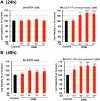
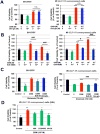
Proglucagon-derived peptides, especially glucagon-like peptide-1 (GLP-1) and its long-acting mimetics, have exhibited neuroprotective effects in animal models of stroke. Several of these peptides are in clinical trials for stroke. Oxyntomodulin (OXM) is a proglucagon-derived peptide that co-activates the GLP-1 receptor (GLP-1R) and the glucagon receptor (GCGR). The neuroprotective action of OXM, however, has not been thoroughly investigated. In this study, the neuroprotective effect of OXM was first examined in human neuroblastoma (SH-SY5Y) cells and rat primary cortical neurons. GLP-1R and GCGR antagonists, and inhibitors of various signaling pathways were used in cell culture to characterize the mechanisms of action of OXM. To evaluate translation in vivo, OXM-mediated neuroprotection was assessed in a 60-min, transient middle cerebral artery occlusion (MCAo) rat model of stroke. We found that OXM dose- and time-dependently increased cell viability and protected cells from glutamate toxicity and oxidative stress. These neuroprotective actions of OXM were mainly mediated through the GLP-1R. OXM induced intracellular cAMP production and activated cAMP-response element-binding protein (CREB). Furthermore, inhibition of the PKA and MAPK pathways, but not inhibition of the PI3K pathway, significantly attenuated the OXM neuroprotective actions. Intracerebroventricular administration of OXM significantly reduced cerebral infarct size and improved locomotor activities in MCAo stroke rats. Therefore, we conclude that OXM is neuroprotective against ischemic brain injury. The mechanisms of action involve induction of intracellular cAMP, activation of PKA and MAPK pathways and phosphorylation of CREB.
Keywords: Glucagon receptor; Glucagon-like peptide-1 receptor; Glutamate excitotoxicity; Neuroprotection; Oxidative stress; Oxyntomodulin; Stroke.
Published by Elsevier Inc.
Figures



Similar articles
-
Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure.Gastroenterology. 2004 Aug;127(2):546-58. doi: 10.1053/j.gastro.2004.04.063. Gastroenterology. 2004. PMID: 15300587
-
The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances beta-cell function but does not inhibit gastric emptying in mice.Endocrinology. 2008 Nov;149(11):5670-8. doi: 10.1210/en.2008-0336. Epub 2008 Jul 31. Endocrinology. 2008. PMID: 18669601
-
Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells.J Neurochem. 2010 Jun;113(6):1621-31. doi: 10.1111/j.1471-4159.2010.06731.x. Epub 2010 Apr 2. J Neurochem. 2010. PMID: 20374430 Free PMC article.
-
Central pre-proglucagon derived peptides: opportunities for treatment of obesity.Curr Pharm Des. 2003;9(17):1373-82. doi: 10.2174/1381612033454775. Curr Pharm Des. 2003. PMID: 12769729 Review.
-
Emerging roles of oxyntomodulin-based glucagon-like peptide-1/glucagon co-agonist analogs in diabetes and obesity.Peptides. 2023 Apr;162:170955. doi: 10.1016/j.peptides.2023.170955. Epub 2023 Jan 18. Peptides. 2023. PMID: 36669563 Review.
Cited by
-
The neuroprotective effects of glucagon-like peptide 1 in Alzheimer's and Parkinson's disease: An in-depth review.Front Neurosci. 2022 Sep 1;16:970925. doi: 10.3389/fnins.2022.970925. eCollection 2022. Front Neurosci. 2022. PMID: 36117625 Free PMC article. Review.
-
The metabolite GLP-1 (9-36) is neuroprotective and anti-inflammatory in cellular models of neurodegeneration.J Neurochem. 2021 Dec;159(5):867-886. doi: 10.1111/jnc.15521. Epub 2021 Oct 21. J Neurochem. 2021. PMID: 34569615 Free PMC article.
-
The role of amphipathic and cationic helical peptides in Parkinson's disease.Protein Sci. 2025 Jan;34(1):e70020. doi: 10.1002/pro.70020. Protein Sci. 2025. PMID: 39720890 Free PMC article. Review.
-
A New Treatment Strategy for Parkinson's Disease through the Gut-Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway.Cell Transplant. 2017 Sep;26(9):1560-1571. doi: 10.1177/0963689717721234. Cell Transplant. 2017. PMID: 29113464 Free PMC article. Review.
-
Promising biomarkers and therapeutic targets for the management of Parkinson's disease: recent advancements and contemporary research.Metab Brain Dis. 2023 Mar;38(3):873-919. doi: 10.1007/s11011-023-01180-z. Epub 2023 Feb 20. Metab Brain Dis. 2023. PMID: 36807081 Review.
References
-
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. - PubMed
-
- Armstead WM, Kiessling JW, Cines DB, Higazi AA. Glucagon protects against impaired NMDA-mediated cerebrovasodilation and cerebral autoregulation during hypotension after brain injury by activating cAMP protein kinase A and inhibiting upregulation of tPA. J Neurotrauma. 2011;28:451–457. - PMC - PubMed
-
- Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: mechanisms of action. Drug Discov Today. 2016;21:802–818. - PubMed
-
- Bagger JI, Holst JJ, Hartmann B, Andersen B, Knop FK, Vilsbøll T. Effect of Oxyntomodulin, Glucagon, GLP-1, and Combined Glucagon +GLP-1 Infusion on Food Intake, Appetite, and Resting Energy Expenditure. J Clin Endocrinol Metab. 2015;100:4541–4552. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

