Genomics of Ovarian Cancer Progression Reveals Diverse Metastatic Trajectories Including Intraepithelial Metastasis to the Fallopian Tube
- PMID: 27856443
- PMCID: PMC5164915
- DOI: 10.1158/2159-8290.CD-16-0607
Genomics of Ovarian Cancer Progression Reveals Diverse Metastatic Trajectories Including Intraepithelial Metastasis to the Fallopian Tube
Abstract
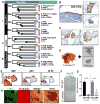
Accumulating evidence has supported the fallopian tube rather than the ovary as the origin for high-grade serous ovarian cancer (HGSOC). To understand the relationship between putative precursor lesions and metastatic tumors, we performed whole-exome sequencing on specimens from eight HGSOC patient progression series consisting of serous tubal intraepithelial carcinomas (STIC), invasive fallopian tube lesions, invasive ovarian lesions, and omental metastases. Integration of copy number and somatic mutations revealed patient-specific patterns with similar mutational signatures and copy-number variation profiles across all anatomic sites, suggesting that genomic instability is an early event in HGSOC. Phylogenetic analyses supported STIC as precursor lesions in half of our patient cohort, but also identified STIC as metastases in 2 patients. Ex vivo assays revealed that HGSOC spheroids can implant in the fallopian tube epithelium and mimic STIC lesions. That STIC may represent metastases calls into question the assumption that STIC are always indicative of primary fallopian tube cancers.
Significance: We find that the putative precursor lesions for HGSOC, STIC, possess most of the genomic aberrations present in advanced cancers. In addition, a proportion of STIC represent intraepithelial metastases to the fallopian tube rather than the origin of HGSOC. Cancer Discov; 6(12); 1342-51. ©2016 AACR.See related commentary by Swisher et al., p. 1309This article is highlighted in the In This Issue feature, p. 1293.
©2016 American Association for Cancer Research.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures



Comment in
-
Culprit or Bystander? The Role of the Fallopian Tube in "Ovarian" High-Grade Serous Carcinoma.Cancer Discov. 2016 Dec;6(12):1309-1311. doi: 10.1158/2159-8290.CD-16-1197. Cancer Discov. 2016. PMID: 27920138
References
-
- Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–94. - PubMed
-
- Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. Journal of Pathology. 2001;195(4):451–6. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

