RasGRF Couples Nox4-Dependent Endoplasmic Reticulum Signaling to Ras
- PMID: 27856453
- PMCID: PMC5222703
- DOI: 10.1161/ATVBAHA.116.307922
RasGRF Couples Nox4-Dependent Endoplasmic Reticulum Signaling to Ras
Abstract
Objectives: In response to endoplasmic reticulum (ER) stress, endothelial cells initiate corrective pathways such as the unfolded protein response. Recent studies suggest that reactive oxygen species produced on the ER may participate in homeostatic signaling through Ras in response to ER stress. We sought to identify mechanisms responsible for this focal signaling pathway.
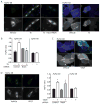
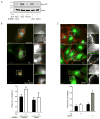
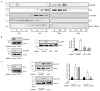
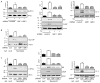
Approach and results: In endothelial cells, we found that ER stress induced by tunicamycin activates the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase Nox4 focally on the ER surface but not on the plasma membrane. Ras activation is also restricted to the ER, occurs downstream of Nox4, and is required for activation of the unfolded protein response. In contrast, treatment with the growth factor VEGF (vascular endothelial growth factor) results in Ras activation and reactive oxygen species production confined instead to the plasma membrane and not to the ER, demonstrating local coupling of reactive oxygen species and Ras signals. We further identify the calcium-responsive, ER-resident guanyl exchange factors RasGRF1 and RasGRF2 as novel upstream mediators linking Nox4 with Ras activation in response to ER stress. Oxidation of the sarcoendoplasmic reticulum calcium ATPase and increases in cytosolic calcium caused by ER stress are blocked by Nox4 knockdown, and reduction in cytosolic free calcium prevents both Ras activation and the unfolded protein response.
Conclusions: ER stress triggers a localized signaling module on the ER surface involving Nox4-dependent calcium mobilization, which directs local Ras activation through ER-associated, calcium-responsive RasGRF.
Keywords: RasGRF1; apoptosis; autophagy; calcium; tunicamycin.
© 2016 American Heart Association, Inc.
Figures

References
-
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

