Chromoblastomycosis
- PMID: 27856522
- PMCID: PMC5217794
- DOI: 10.1128/CMR.00032-16
Chromoblastomycosis
Abstract
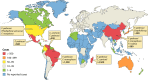
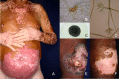
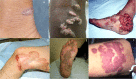
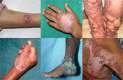
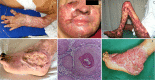
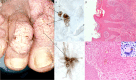
Chromoblastomycosis (CBM), also known as chromomycosis, is one of the most prevalent implantation fungal infections, being the most common of the gamut of mycoses caused by melanized or brown-pigmented fungi. CBM is mainly a tropical or subtropical disease that may affect individuals with certain risk factors around the world. The following characteristics are associated with this disease: (i) traumatic inoculation by implantation from an environmental source, leading to an initial cutaneous lesion at the inoculation site; (ii) chronic and progressive cutaneous and subcutaneous tissular involvement associated with fibrotic and granulomatous reactions associated with microabscesses and often with tissue proliferation; (iii) a nonprotective T helper type 2 (Th2) immune response with ineffective humoral involvement; and (iv) the presence of muriform (sclerotic) cells embedded in the affected tissue. CBM lesions are clinically polymorphic and are commonly misdiagnosed as various other infectious and noninfectious diseases. In its more severe clinical forms, CBM may cause an incapacity for labor due to fibrotic sequelae and also due to a series of clinical complications, and if not recognized at an early stage, this disease can be refractory to antifungal therapy.
Keywords: black fungi; chromoblastomycosis; chromomycosis; melanized fungi; muriform (sclerotic) cells; neglected disease.
Copyright © 2016 American Society for Microbiology.
Figures


















References
-
- Savioli L, Daumerie D, World Health Organization Department of Control of Neglected Tropical Diseases . 2013. Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases. World Health Organization, Geneva, Switzerland.
-
- Reiss H, Shadomy HJ, Lyon GM III. 2012. Fundamental medical mycology. Wiley-Blackwell, Hoboken, NJ.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

