Loss of AKAP150 promotes pathological remodelling and heart failure propensity by disrupting calcium cycling and contractile reserve
- PMID: 27856611
- PMCID: PMC5340143
- DOI: 10.1093/cvr/cvw221
Loss of AKAP150 promotes pathological remodelling and heart failure propensity by disrupting calcium cycling and contractile reserve
Abstract
Aims: Impaired Ca2 + cycling and myocyte contractility are a hallmark of heart failure triggered by pathological stress such as hemodynamic overload. The A-Kinase anchoring protein AKAP150 has been shown to coordinate key aspects of adrenergic regulation of Ca2+ cycling and excitation-contraction in cardiomyocytes. However, the role of the AKAP150 signalling complexes in the pathogenesis of heart failure has not been investigated.
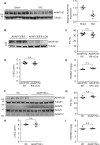
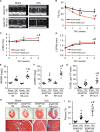
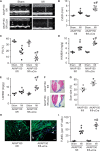
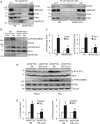
Methods and results: Here we examined how AKAP150 signalling complexes impact Ca2+ cycling, myocyte contractility, and heart failure susceptibility following pathological stress. We detected a significant reduction of AKAP150 expression in the failing mouse heart induced by pressure overload. Importantly, cardiac-specific AKAP150 knockout mice were predisposed to develop dilated cardiomyopathy with severe cardiac dysfunction and fibrosis after pressure overload. Loss of AKAP150 also promoted pathological remodelling and heart failure progression following myocardial infarction. However, ablation of AKAP150 did not affect calcineurin-nuclear factor of activated T cells signalling in cardiomyocytes or pressure overload- or agonist-induced cardiac hypertrophy. Immunoprecipitation studies showed that AKAP150 was associated with SERCA2, phospholamban, and ryanodine receptor-2, providing a targeted control of sarcoplasmic reticulum Ca2+ regulatory proteins. Mechanistically, loss of AKAP150 led to impaired Ca2+ cycling and reduced myocyte contractility reserve following adrenergic stimulation or pressure overload.
Conclusions: These findings define a critical role for AKAP150 in regulating Ca2+ cycling and myocardial ionotropy following pathological stress, suggesting the AKAP150 signalling pathway may serve as a novel therapeutic target for heart failure.
Keywords: AKAP150; Cardiac hypertrophy; Contractility; Heart failure; Pathological remodelling.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2016. For Permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
A gene therapeutic approach to inhibit calcium and integrin binding protein 1 ameliorates maladaptive remodelling in pressure overload.Cardiovasc Res. 2019 Jan 1;115(1):71-82. doi: 10.1093/cvr/cvy154. Cardiovasc Res. 2019. PMID: 29931050
-
Early calcium handling imbalance in pressure overload-induced heart failure with nearly normal left ventricular ejection fraction.Biochim Biophys Acta Mol Basis Dis. 2019 Jan;1865(1):230-242. doi: 10.1016/j.bbadis.2018.08.005. Epub 2018 Aug 4. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30463691
-
Hypoxia-responsive zinc finger E-box-binding homeobox 2 (ZEB2) regulates a network of calcium-handling genes in the injured heart.Cardiovasc Res. 2024 Dec 4;120(15):1869-1883. doi: 10.1093/cvr/cvae163. Cardiovasc Res. 2024. PMID: 39308239 Free PMC article.
-
Calcium-regulated transcriptional pathways in the normal and pathologic heart.IUBMB Life. 2011 Oct;63(10):847-55. doi: 10.1002/iub.545. Epub 2011 Sep 7. IUBMB Life. 2011. PMID: 21901815 Review.
-
Altered sarcoplasmic reticulum calcium cycling--targets for heart failure therapy.Nat Rev Cardiol. 2012 Dec;9(12):717-33. doi: 10.1038/nrcardio.2012.145. Epub 2012 Oct 23. Nat Rev Cardiol. 2012. PMID: 23090087 Free PMC article. Review.
Cited by
-
Calcineurin-AKAP interactions: therapeutic targeting of a pleiotropic enzyme with a little help from its friends.J Physiol. 2020 Jul;598(14):3029-3042. doi: 10.1113/JP276756. Epub 2018 Dec 26. J Physiol. 2020. PMID: 30488951 Free PMC article. Review.
-
A-Kinase Anchoring Protein-Lbc: A Molecular Scaffold Involved in Cardiac Protection.J Cardiovasc Dev Dis. 2018 Feb 8;5(1):12. doi: 10.3390/jcdd5010012. J Cardiovasc Dev Dis. 2018. PMID: 29419761 Free PMC article. Review.
-
Function of Adenylyl Cyclase in Heart: the AKAP Connection.J Cardiovasc Dev Dis. 2018 Jan 16;5(1):2. doi: 10.3390/jcdd5010002. J Cardiovasc Dev Dis. 2018. PMID: 29367580 Free PMC article. Review.
-
Serum Proteins Predict Treatment-Related Cardiomyopathy Among Survivors of Childhood Cancer.JACC CardioOncol. 2024 Dec 3;7(1):56-67. doi: 10.1016/j.jaccao.2024.10.004. eCollection 2025 Jan. JACC CardioOncol. 2024. PMID: 39896123 Free PMC article.
-
Cardiac function modulation depends on the A-kinase anchoring protein complex.J Cell Mol Med. 2019 Nov;23(11):7170-7179. doi: 10.1111/jcmm.14659. Epub 2019 Sep 11. J Cell Mol Med. 2019. PMID: 31512389 Free PMC article. Review.
References
-
- Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science 2003;300:1530–1532. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature 2002;415:198–205. - PubMed
-
- Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 2005;66:12–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

