Effects of Vitamin D Receptor Activation and Dietary Sodium Restriction on Residual Albuminuria in CKD: The ViRTUE-CKD Trial
- PMID: 27856633
- PMCID: PMC5373450
- DOI: 10.1681/ASN.2016040407
Effects of Vitamin D Receptor Activation and Dietary Sodium Restriction on Residual Albuminuria in CKD: The ViRTUE-CKD Trial
Abstract
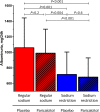
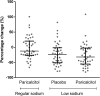
Reduction of residual albuminuria during single-agent renin-angiotensin-aldosterone blockade is accompanied by improved cardiorenal outcomes in CKD. We studied the individual and combined effects of the vitamin D receptor activator paricalcitol (PARI) and dietary sodium restriction on residual albuminuria in CKD. In a multicenter, randomized, placebo (PLAC)-controlled, crossover trial, 45 patients with nondiabetic CKD stages 1-3 and albuminuria >300 mg/24 h despite ramipril at 10 mg/d and BP<140/90 mmHg were treated for four 8-week periods with PARI (2 μg/d) or PLAC, each combined with a low-sodium (LS) or regular sodium (RS) diet. We analyzed the treatment effect by linear mixed effect models for repeated measurements. In the intention-to-treat analysis, albuminuria (geometric mean) was 1060 (95% confidence interval, 778 to 1443) mg/24 h during RS + PLAC and 990 (95% confidence interval, 755 to 1299) mg/24 h during RS + PARI (P=0.20 versus RS + PLAC). LS + PLAC reduced albuminuria to 717 (95% confidence interval, 512 to 1005) mg/24 h (P<0.001 versus RS + PLAC), and LS + PARI reduced albuminuria to 683 (95% confidence interval, 502 to 929) mg/24 h (P<0.001 versus RS + PLAC). The reduction by PARI beyond the effect of LS was nonsignificant (P=0.60). In the per-protocol analysis restricted to participants with ≥95% compliance with study medication, PARI did provide further albuminuria reduction (P=0.04 LS + PARI versus LS + PLAC). Dietary adherence was good as reflected by urinary excretion of 174±64 mmol Na+ per day in the combined RS groups and 108±61 mmol Na+ per day in the LS groups (P<0.001). In conclusion, moderate dietary sodium restriction substantially reduced residual albuminuria during fixed dose angiotensin-converting enzyme inhibition. The additional effect of PARI was small and nonsignificant.
Keywords: VDRA; albuminuria; chronic kidney disease; dietary sodium restriction; paricalcitol; randomized-controlled trial.
Copyright © 2017 by the American Society of Nephrology.
Figures


Comment in
-
Low Sodium Diet, Vitamin D, or Both for RAASi-Resistant, Residual, Proteinuria in CKD? The ViRTUE Trial Points the Way Forward but Is Not the Last Word.J Am Soc Nephrol. 2017 Apr;28(4):1016-1019. doi: 10.1681/ASN.2016121321. Epub 2017 Feb 28. J Am Soc Nephrol. 2017. PMID: 28246129 Free PMC article. No abstract available.
References
-
- Zucchelli P, Zuccalà A, Borghi M, Fusaroli M, Sasdelli M, Stallone C, Sanna G, Gaggi R: Long-term comparison between captopril and nifedipine in the progression of renal insufficiency. Kidney Int 42: 452–458, 1992 - PubMed
-
- Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P: Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The angiotensin-converting-enzyme inhibition in progressive renal insufficiency study group. N Engl J Med 334: 939–945, 1996 - PubMed
-
- GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 349: 1857–1863, 1997 - PubMed
-
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G: Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 342: 145–153, 2000 - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

