Nasal high flow reduces dead space
- PMID: 27856714
- PMCID: PMC5283847
- DOI: 10.1152/japplphysiol.00584.2016
Nasal high flow reduces dead space
Abstract
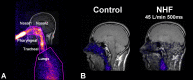
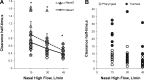
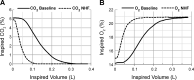
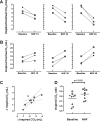
Recent studies show that nasal high flow (NHF) therapy can support ventilation in patients with acute or chronic respiratory disorders. Clearance of dead space has been suggested as being the key mechanism of respiratory support with NHF therapy. The hypothesis of this study was that NHF in a dose-dependent manner can clear dead space of the upper airways from expired air and decrease rebreathing. The randomized crossover study involved 10 volunteers using scintigraphy with 81mKrypton (81mKr) gas during a breath-holding maneuver with closed mouth and in 3 nasally breathing tracheotomized patients by volumetric capnography and oximetry through sampling CO2 and O2 in the trachea and measuring the inspired volume with inductance plethysmography following NHF rates of 15, 30, and 45 l/min. The scintigraphy revealed a decrease in 81mKr gas clearance half-time with an increase of NHF in the nasal cavities [Pearson's correlation coefficient cc = -0.55, P < 0.01], the pharynx (cc = -0.41, P < 0.01), and the trachea (cc = -0.51, P < 0.01). Clearance rates in nasal cavities derived from time constants and MRI-measured volumes were 40.6 ± 12.3 (SD), 52.5 ± 17.7, and 72.9 ± 21.3 ml/s during NHF (15, 30, and 45 l/min, respectively). Measurement of inspired gases in the trachea showed an NHF-dependent decrease of inspired CO2 that correlated with an increase of inspired O2 (cc = -0.77, P < 0.05). NHF clears the upper airways of expired air, which reduces dead space by a decrease of rebreathing making ventilation more efficient. The dead space clearance is flow and time dependent, and it may extend below the soft palate.
New & noteworthy: Clearance of expired air in upper airways by nasal high flow (NHF) can be extended below the soft palate and de facto causes a reduction of dead space. Using scintigraphy, the authors found a relationship between NHF, time, and clearance. Direct measurement of CO2 and O2 in the trachea confirmed a reduction of rebreathing, providing the actual data on inspired gases, and this can be used for the assessment of other forms of respiratory support.
Keywords: Krypton; dead space; nasal high flow; rebreathing; respiratory support; upper airways.
Copyright © 2017 the American Physiological Society.
Conflict of interest statement
W. Möller received research grants from Pari GmbH, Germany, for studying nasal aerosolized drug delivery, and from Fisher & Paykel Healthcare, New Zealand, for studying the role of nasal high flow in dead space clearance. G. Nilius received research grants from Fisher & Paykel Healthcare, ResMed, Respironics Inc., Philips, Weimann, and Heinen & Löwenstein. S. Feng and S. Tatkov are employees of Fisher & Paykel Healthcare, New Zealand. All other authors declare no conflicts of interest.
Figures
References
-
- Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Med J Aust 188: 657–659, 2008. - PubMed
-
- Fraser JF, Spooner AJ, Dunster KR, Anstey CM, Corley A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax 71: 759–761, 2016. doi: 10.1136/thoraxjnl-2015-207962. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

