Trypanosoma brucei metabolite indolepyruvate decreases HIF-1α and glycolysis in macrophages as a mechanism of innate immune evasion
- PMID: 27856732
- PMCID: PMC5137691
- DOI: 10.1073/pnas.1608221113
Trypanosoma brucei metabolite indolepyruvate decreases HIF-1α and glycolysis in macrophages as a mechanism of innate immune evasion
Abstract
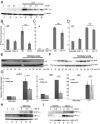
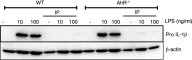
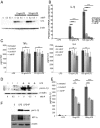
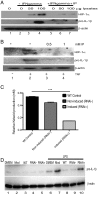
The parasite Trypanasoma brucei causes African trypanosomiasis, known as sleeping sickness in humans and nagana in domestic animals. These diseases are a major burden in the 36 sub-Saharan African countries where the tsetse fly vector is endemic. Untreated trypanosomiasis is fatal and the current treatments are stage-dependent and can be problematic during the meningoencephalitic stage, where no new therapies have been developed in recent years and the current drugs have a low therapeutic index. There is a need for more effective treatments and a better understanding of how these parasites evade the host immune response will help in this regard. The bloodstream form of T. brucei excretes significant amounts of aromatic ketoacids, including indolepyruvate, a transamination product of tryptophan. This study demonstrates that this process is essential in bloodstream forms, is mediated by a specialized isoform of cytoplasmic aminotransferase and, importantly, reveals an immunomodulatory role for indolepyruvate. Indolepyruvate prevents the LPS-induced glycolytic shift in macrophages. This effect is the result of an increase in the hydroxylation and degradation of the transcription factor hypoxia-inducible factor-1α (HIF-1α). The reduction in HIF-1α levels by indolepyruvate, following LPS or trypanosome activation, results in a decrease in production of the proinflammatory cytokine IL-1β. These data demonstrate an important role for indolepyruvate in immune evasion by T. brucei.
Keywords: Trypanosoma brucei; immune evasion; immunometabolism; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

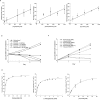
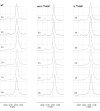
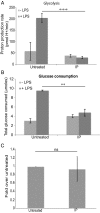
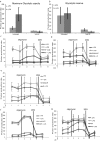


References
-
- Cross GA. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. - PubMed
-
- Pays E, Vanhamme L, Berberof M. Genetic controls for the expression of surface antigens in African trypanosomes. Annu Rev Microbiol. 1994;48:25–52. - PubMed
-
- Morrison LJ, Marcello L, McCulloch R. Antigenic variation in the African trypanosome: Molecular mechanisms and phenotypic complexity. Cell Microbiol. 2009;11(12):1724–1734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

