Global transformation of erythrocyte properties via engagement of an SH2-like sequence in band 3
- PMID: 27856737
- PMCID: PMC5137735
- DOI: 10.1073/pnas.1611904113
Global transformation of erythrocyte properties via engagement of an SH2-like sequence in band 3
Abstract
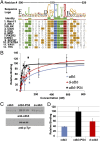
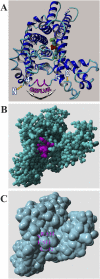
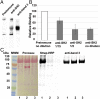
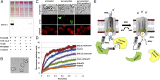
Src homology 2 (SH2) domains are composed of weakly conserved sequences of ∼100 aa that bind phosphotyrosines in signaling proteins and thereby mediate intra- and intermolecular protein-protein interactions. In exploring the mechanism whereby tyrosine phosphorylation of the erythrocyte anion transporter, band 3, triggers membrane destabilization, vesiculation, and fragmentation, we discovered a SH2 signature motif positioned between membrane-spanning helices 4 and 5. Evidence that this exposed cytoplasmic sequence contributes to a functional SH2-like domain is provided by observations that: (i) it contains the most conserved sequence of SH2 domains, GSFLVR; (ii) it binds the tyrosine phosphorylated cytoplasmic domain of band 3 (cdb3-PO4) with Kd = 14 nM; (iii) binding of cdb3-PO4 to erythrocyte membranes is inhibited both by antibodies against the SH2 signature sequence and dephosphorylation of cdb3-PO4; (iv) label transfer experiments demonstrate the covalent transfer of photoactivatable biotin from isolated cdb3-PO4 (but not cdb3) to band 3 in erythrocyte membranes; and (v) phosphorylation-induced binding of cdb3-PO4 to the membrane-spanning domain of band 3 in intact cells causes global changes in membrane properties, including (i) displacement of a glycolytic enzyme complex from the membrane, (ii) inhibition of anion transport, and (iii) rupture of the band 3-ankyrin bridge connecting the spectrin-based cytoskeleton to the membrane. Because SH2-like motifs are not retrieved by normal homology searches for SH2 domains, but can be found in many tyrosine kinase-regulated transport proteins using modified search programs, we suggest that related cases of membrane transport proteins containing similar motifs are widespread in nature where they participate in regulation of cell properties.
Keywords: SH2 domain motif; anion exchanger 1; erythrocyte glycolysis; regulation of transport proteins; tyrosine phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pawson T, Kofler M. Kinome signaling through regulated protein-protein interactions in normal and cancer cells. Curr Opin Cell Biol. 2009;21(2):147–153. - PubMed
-
- Liu BA, Engelmann BW, Nash PD. The language of SH2 domain interactions defines phosphotyrosine-mediated signal transduction. FEBS Lett. 2012;586(17):2597–2605. - PubMed
-
- van den Akker E, Satchwell TJ, Williamson RC, Toye AM. Band 3 multiprotein complexes in the red cell membrane; of mice and men. Blood Cells Mol Dis. 2010;45(1):1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

