All- Trans-Retinoic Acid Augments the Histopathological Outcome of Neuroinflammation and Neurodegeneration in Lupus-Prone MRL/lpr Mice
- PMID: 27856824
- PMCID: PMC5256197
- DOI: 10.1369/0022155416679638
All- Trans-Retinoic Acid Augments the Histopathological Outcome of Neuroinflammation and Neurodegeneration in Lupus-Prone MRL/lpr Mice
Abstract
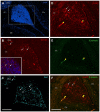
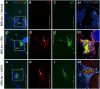
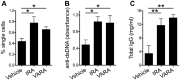
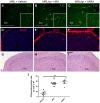
Recently, we demonstrated that treatment with all- trans-retinoic acid (tRA) induced a paradoxical effect on immune activation during the development of autoimmune lupus. Here, we further describe its negative effects on mediating neuroinflammation and neurodegeneration. Female MRL/lpr mice were orally administered tRA or VARA (retinol mixed with 10% tRA) from 6 to 14 weeks of age. Both treatments had a significant effect on brain weight, which correlated with histopathological evidence of focal astrogliosis, meningitis, and ventriculitis. Infiltration of CD138- and Iba1-positve immune cells was observed in the third ventricle and meninges of treated mice that co-labeled with ICAM-1, indicating their inflammatory nature. Increased numbers of circulating plasma cells, autoantibodies, and total IgG were also apparent. IgG and C3 complement deposition in these brain regions were also prominent as was focal astrogliosis surrounding the ventricular lining and meninges. Using Fluoro-Jade staining, we further demonstrate that neuroinflammation was accompanied by neurodegeneration in the cortex of treated mice compared with vehicle controls. These findings indicate that vitamin A exposure exacerbates the immunogenic environment of the brain during the onset of systemic autoimmune disease. Vitamin A may therefore compromise the immuno-privileged nature of the central nervous system under a predisposed immunogenic environment.
Keywords: inflammation; lupus; retinoic acid.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Paradoxical effects of all-trans-retinoic acid on lupus-like disease in the MRL/lpr mouse model.PLoS One. 2015 Mar 16;10(3):e0118176. doi: 10.1371/journal.pone.0118176. eCollection 2015. PLoS One. 2015. PMID: 25775135 Free PMC article.
-
Retinoic acid treatment protects MRL/lpr lupus mice from the development of glomerular disease.Kidney Int. 2004 Sep;66(3):1018-28. doi: 10.1111/j.1523-1755.2004.00850.x. Kidney Int. 2004. PMID: 15327395
-
Neurodegeneration in autoimmune MRL-lpr mice as revealed by Fluoro Jade B staining.Brain Res. 2003 Feb 28;964(2):200-10. doi: 10.1016/s0006-8993(02)03980-x. Brain Res. 2003. PMID: 12576180
-
The central and multiple roles of B cells in lupus pathogenesis.Immunol Rev. 1999 Jun;169:107-21. doi: 10.1111/j.1600-065x.1999.tb01310.x. Immunol Rev. 1999. PMID: 10450512 Review.
-
Use of genetic knockouts to modulate disease expression in a murine model of lupus, MRL/lpr mice.Immunol Res. 2002;25(2):143-53. doi: 10.1385/ir:25:2:143. Immunol Res. 2002. PMID: 11999168 Review.
Cited by
-
Amylin and Secretases in the Pathology and Treatment of Alzheimer's Disease.Biomolecules. 2022 Jul 17;12(7):996. doi: 10.3390/biom12070996. Biomolecules. 2022. PMID: 35883551 Free PMC article. Review.
-
Lactobacillus: Friend or Foe for Systemic Lupus Erythematosus?Front Immunol. 2022 May 23;13:883747. doi: 10.3389/fimmu.2022.883747. eCollection 2022. Front Immunol. 2022. PMID: 35677055 Free PMC article. Review.
-
Treatment of progressive multiple sclerosis with high-dose all-trans retinoic acid - no clear evidence of positive disease modifying effects.Neurol Res Pract. 2021 May 10;3(1):25. doi: 10.1186/s42466-021-00121-4. Neurol Res Pract. 2021. PMID: 33966627 Free PMC article.
-
Intranasal Delivery: Effects on the Neuroimmune Axes and Treatment of Neuroinflammation.Pharmaceutics. 2020 Nov 20;12(11):1120. doi: 10.3390/pharmaceutics12111120. Pharmaceutics. 2020. PMID: 33233734 Free PMC article. Review.
-
Regulation of the germinal center response by nuclear receptors and implications for autoimmune diseases.FEBS J. 2020 Jul;287(14):2866-2890. doi: 10.1111/febs.15312. Epub 2020 Apr 19. FEBS J. 2020. PMID: 32246891 Free PMC article. Review.
References
-
- Boyd RE, Birchmore DA, Kaiser DL, Young AC, Davis JS. Acute effects of steroids on immune complex profile of patients with systemic lupus erythematosus. Correlation of profile with development of target organ involvement. Arthritis Rheum. 1983;26:637–44. - PubMed
-
- Kozora E, Filley CM, Zhang L, Brown MS, Miller DE, Arciniegas DB, Pelzman JL, West SG. Immune function and brain abnormalities in patients with systemic lupus erythematosus without overt neuropsychiatric manifestations. Lupus. 2012;21:402–11. - PubMed
-
- Sthoeger Z, Zinger H, Dekel B, Arditi F, Reisner Y, Mozes E. Lupus manifestations in severe combined immunodeficient (SCID) mice and in human/mouse radiation chimeras. J Clin Immunol. 2003;23:91–9. - PubMed
-
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

