An elastic element in the protocadherin-15 tip link of the inner ear
- PMID: 27857071
- PMCID: PMC5120219
- DOI: 10.1038/ncomms13458
An elastic element in the protocadherin-15 tip link of the inner ear
Abstract
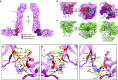
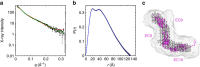
Tip link filaments convey force and gate inner-ear hair-cell transduction channels to mediate perception of sound and head movements. Cadherin-23 and protocadherin-15 form tip links through a calcium-dependent interaction of their extracellular domains made of multiple extracellular cadherin (EC) repeats. These repeats are structurally similar, but not identical in sequence, often featuring linkers with conserved calcium-binding sites that confer mechanical strength to them. Here we present the X-ray crystal structures of human protocadherin-15 EC8-EC10 and mouse EC9-EC10, which show an EC8-9 canonical-like calcium-binding linker, and an EC9-10 calcium-free linker that alters the linear arrangement of EC repeats. Molecular dynamics simulations and small-angle X-ray scattering experiments support this non-linear conformation. Simulations also suggest that unbending of EC9-10 confers some elasticity to otherwise rigid tip links. The new structure provides a first view of protocadherin-15's non-canonical EC linkers and suggests how they may function in inner-ear mechanotransduction, with implications for other cadherins.
Figures






References
-
- Hudspeth A. J. Integrating the active process of hair cells with cochlear function. Nat. Rev. Neurosci. 15, 600–614 (2014). - PubMed
-
- Pickles J. O., Comis S. D. & Osborne M. P. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 15, 103–112 (1984). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

