Dormant cancer cells accumulate high protoporphyrin IX levels and are sensitive to 5-aminolevulinic acid-based photodynamic therapy
- PMID: 27857072
- PMCID: PMC5114660
- DOI: 10.1038/srep36478
Dormant cancer cells accumulate high protoporphyrin IX levels and are sensitive to 5-aminolevulinic acid-based photodynamic therapy
Abstract
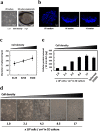
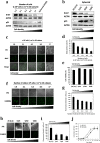
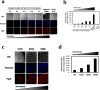
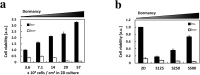
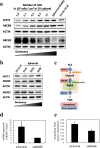
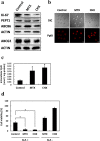
Photodynamic therapy (PDT) and diagnosis (PDD) using 5-aminolevulinic acid (ALA) to drive the production of an intracellular photosensitizer, protoporphyrin IX (PpIX), are in common clinical use. However, the tendency to accumulate PpIX is not well understood. Patients with cancer can develop recurrent metastatic disease with latency periods. This pause can be explained by cancer dormancy. Here we created uniformly sized PC-3 prostate cancer spheroids using a 3D culture plate (EZSPHERE). We demonstrated that cancer cells exhibited dormancy in a cell density-dependent manner not only in spheroids but also in 2D culture. Dormant cancer cells accumulated high PpIX levels and were sensitive to ALA-PDT. In dormant cancer cells, transporter expressions of PEPT1, ALA importer, and ABCB6, an intermediate porphyrin transporter, were upregulated and that of ABCG2, a PpIX exporter, was downregulated. PpIX accumulation and ALA-PDT cytotoxicity were enhanced by G0/G1-phase arrestors in non-dormant cancer cells. Our results demonstrate that ALA-PDT would be an effective approach for dormant cancer cells and can be enhanced by combining with a cell-growth inhibitor.
Conflict of interest statement
SBI Pharma CO., Ltd., provided support in the form of salaries for authors M.N. and T.T., but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures
References
-
- Krammer B. & Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem. Photobiol. Sci. 7, 283–289 (2008). - PubMed
-
- Kennedy J. C. & Pottier R. H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B 14, 275–292 (1992). - PubMed
-
- Tabata K., Ogura S. & Okura I. Photodynamic efficiency of protoporphyrin IX: comparison of endogenous protoporphyrin IXinduced by 5-aminolevulinic acid and exogenous porphyrin IX. Photochem. Photobiol. 66, 842–846 (1997).
-
- Stummer W. et al.. Fluorescence-guided surgery with 5-aminolevulinicacid for resection of malignant glioma: a randomised controlledmulticentre phase III trial. Lancet Oncol 7, 392–401 (2006). - PubMed
-
- Inoue K. et al.. Comparison between intravesical and oraladministration of 5-aminolevulinic acid in the clinical bene-fit of photodynamic diagnosis for non-muscle invasive bladdercancer. Cancer. 118, 1062–1074 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

