3D visualization of additive occlusion and tunable full-spectrum fluorescence in calcite
- PMID: 27857076
- PMCID: PMC5120221
- DOI: 10.1038/ncomms13524
3D visualization of additive occlusion and tunable full-spectrum fluorescence in calcite
Abstract
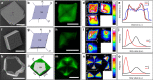
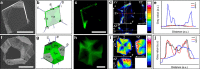
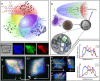
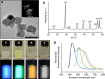
From biomineralization to synthesis, organic additives provide an effective means of controlling crystallization processes. There is growing evidence that these additives are often occluded within the crystal lattice. This promises an elegant means of creating nanocomposites and tuning physical properties. Here we use the incorporation of sulfonated fluorescent dyes to gain new understanding of additive occlusion in calcite (CaCO3), and to link morphological changes to occlusion mechanisms. We demonstrate that these additives are incorporated within specific zones, as defined by the growth conditions, and show how occlusion can govern changes in crystal shape. Fluorescence spectroscopy and lifetime imaging microscopy also show that the dyes experience unique local environments within different zones. Our strategy is then extended to simultaneously incorporate mixtures of dyes, whose fluorescence cascade creates calcite nanoparticles that fluoresce white. This offers a simple strategy for generating biocompatible and stable fluorescent nanoparticles whose output can be tuned as required.
Figures




References
-
- Ramamurthy V. & Eaton D. F. Perspectives on solid-state host-guest assemblies. Chem. Mater. 6, 1128–1136 (1994).
-
- Mitra T. et al. Molecular shape sorting using molecular organic cages. Nat. Chem. 5, 276–281 (2013). - PubMed
-
- Chughtai A. H., Ahmad N., Younus H. A., Laypkov A. & Verpoort F. Metal-organic frameworks: versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 44, 6804–6849 (2015). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

