Inhibiting MDSC differentiation from bone marrow with phytochemical polyacetylenes drastically impairs tumor metastasis
- PMID: 27857157
- PMCID: PMC5114612
- DOI: 10.1038/srep36663
Inhibiting MDSC differentiation from bone marrow with phytochemical polyacetylenes drastically impairs tumor metastasis
Abstract
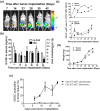
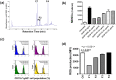
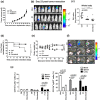
Myeloid-derived suppressor cells (MDSCs) are implicated in the promotion of tumor metastasis by protecting metastatic cancerous cells from immune surveillance and have thus been suggested as novel targets for cancer therapy. We demonstrate here that oral feeding with polyacetylenic glycosides (BP-E-F1) from the medicinal plant Bidens pilosa effectively suppresses tumor metastasis and inhibits tumor-induced accumulation of granulocytic (g) MDSCs, but does not result in body weight loss in a mouse mammary tumor-resection model. BP-E-F1 is further demonstrated to exert its anti-metastasis activity through inhibiting the differentiation and function of gMDSCs. Pharmacokinetic and mechanistic studies reveal that BP-E-F1 suppresses the differentiation of gMDSCs via the inhibition of a tumor-derived, G-CSF-induced signaling pathway in bone marrow cells of test mice. Taken together, our findings suggest that specific plant polyacetylenic glycosides that target gMDSC differentiation by communicating with bone marrow cells may hence be seriously considered for potential application as botanical drugs against metastatic cancers.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

