Magnetic resonance imaging spectrum of perinatal hypoxic-ischemic brain injury
- PMID: 27857456
- PMCID: PMC5036328
- DOI: 10.4103/0971-3026.190421
Magnetic resonance imaging spectrum of perinatal hypoxic-ischemic brain injury
Erratum in
-
Erratum: Magnetic resonance imaging spectrum of perinatal hypoxic-ischemic brain injury.Indian J Radiol Imaging. 2016 Oct-Dec;26(4):530. doi: 10.4103/0971-3026.195815. Indian J Radiol Imaging. 2016. PMID: 28104955 Free PMC article.
Abstract
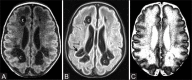
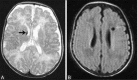
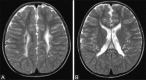
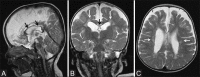
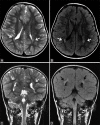
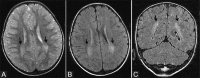
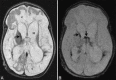
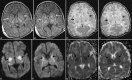
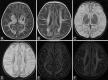
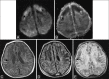
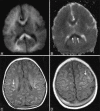
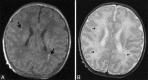
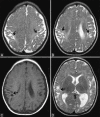
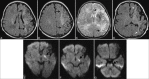
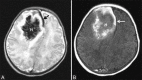
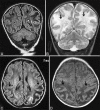
Perinatal hypoxic-ischemic brain injury results in neonatal hypoxic-ischemic encephalopathy and serious long-term neurodevelopmental sequelae. Magnetic resonance imaging (MRI) of the brain is an ideal and safe imaging modality for suspected hypoxic-ischemic injury. The pattern of injury depends on brain maturity at the time of insult, severity of hypotension, and duration of insult. Time of imaging after the insult influences the imaging findings. Mild to moderate hypoperfusion results in germinal matrix hemorrhages and periventricular leukomalacia in preterm neonates and parasagittal watershed territory infarcts in full-term neonates. Severe insult preferentially damages the deep gray matter in both term and preterm infants. However, associated frequent perirolandic injury is seen in term neonates. MRI is useful in establishing the clinical diagnosis, assessing the severity of injury, and thereby prognosticating the outcome. Familiarity with imaging spectrum and insight into factors affecting the injury will enlighten the radiologist to provide an appropriate diagnosis.
Keywords: Cortical highlighting; germinal matrix hemorrhage; hypoxic ischemic encephalopathy; hypoxic ischemic injury; periventricular leukomalacia.
Figures





References
-
- Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95. - PubMed
-
- Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. - PubMed
-
- Vannucci RC, Perlman JM. Interventions for Perinatal Hypoxic-Ischemic Encephalopathy. Pediatrics. 1997;100:1004–114. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

