CT manifestations of small bowel ischemia due to impaired venous drainage-with a correlation of pathologic findings
- PMID: 27857460
- PMCID: PMC5036332
- DOI: 10.4103/0971-3026.190426
CT manifestations of small bowel ischemia due to impaired venous drainage-with a correlation of pathologic findings
Abstract
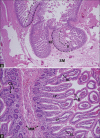
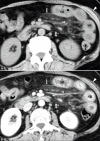
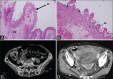
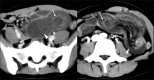
Acute abdominal pain may result from a wide variety of medical and surgical diseases. One of these diseases is small bowel ischemia, which may result in a catastrophic outcome if not recognized and treated promptly. Computed tomography (CT) by its faster image acquisition, thinner collimation, high resolution, and multiplanar reformatted images has become the most important imaging modality in evaluating the acute abdominal conditions. In this article, the author presents a description of the histology of the small bowel, pathophysiology of small bowel change, and a correlation of the pathologic and CT findings of the small bowel injuries due to impaired venous drainage. A convincing correlation of the microscopic mucosal condition with the enhancement pattern of the thickened small bowel wall on CT is useful in definitely describing the mucosal viability.
Keywords: Computed tomography; Intestines; Ischemia; Small bowel.
Figures








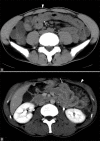
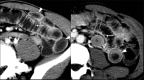
References
-
- Wiesner W, Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226:635–50. - PubMed
-
- Federle MP, Chun G, Jeffrey RB, Rayor R. Computed tomographic findings in bowel infarction. AJR Am J Roentgenol. 1984;142:91–5. - PubMed
-
- Alpern MB, Glazer GM, Francis IR. Ischemic or infracted bowel: CT findings. Radiology. 1988;166:149–52. - PubMed
-
- Bartnicke BJ, Balfe DM. CT appearance of intestinal ischemia and intramural hemorrhage. RadiolClin North Am. 1994;32:845–60. - PubMed
-
- Klein HM, Lensing R, Klosterhalfen B, Tons C, RW Gunther. Diagnostic imaging of mesenteric infarction. Radiology. 1995;197:79–82. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

