Comparative study of the ion flux pathway in stator units of proton- and sodium-driven flagellar motors
- PMID: 27857578
- PMCID: PMC5036635
- DOI: 10.2142/biophysics.5.45
Comparative study of the ion flux pathway in stator units of proton- and sodium-driven flagellar motors
Abstract
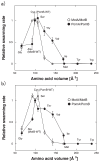
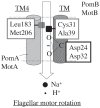
Flagellar motor proteins, MotA/B and PomA/B, are essential for the motility of Escherichia coli and Vibrio alginolyticus, respectively. Those complexes work as a H+ and a Na+ channel, respectively and play important roles in torque generation as the stators of the flagellar motors. Although Asp32 of MotB and Asp24 of PomB are believed to function as ion binding site(s), the ion flux pathway from the periplasm to the cytoplasm is still unclear. Conserved residues, Ala39 of MotB and Cys31 of PomB, are located on the same sides as Asp32 of MotB and Asp24 of PomB, respectively, in a helical wheel diagram. In this study, a series of mutations were introduced into the Ala39 residue of MotB and the Cys31 residue of PomB. The motility of mutant cells were markedly decreased as the volume of the side chain increased. The loss of function due to the MotB(A39V) and PomB(L28A/C31A) mutations was suppressed by mutations of MotA(M206S) and PomA(L183F), respectively, and the increase in the volume caused by the MotB(A39V) mutation was close to the decrease in the volume caused by the MotA(M206S) mutation. These results demonstrate that Ala39 of MotB and Cys31 of PomB form part of the ion flux pathway and pore with Met206 of MotA and Leu183 of PomA in the MotA/B and PomA/B stator units, respectively.
Keywords: bacteria locomotion; ion flux; membrane protein; molecular motor; motility; taxis.
Figures




Similar articles
-
Ion-coupling determinants of Na+-driven and H+-driven flagellar motors.J Mol Biol. 2003 Mar 21;327(2):453-63. doi: 10.1016/s0022-2836(03)00096-2. J Mol Biol. 2003. PMID: 12628250
-
A conserved residue, PomB-F22, in the transmembrane segment of the flagellar stator complex, has a critical role in conducting ions and generating torque.Microbiology (Reading). 2011 Aug;157(Pt 8):2422-2432. doi: 10.1099/mic.0.048488-0. Epub 2011 Jun 2. Microbiology (Reading). 2011. PMID: 21636648
-
Hybrid motor with H(+)- and Na(+)-driven components can rotate Vibrio polar flagella by using sodium ions.J Bacteriol. 1999 Oct;181(20):6332-8. doi: 10.1128/JB.181.20.6332-6338.1999. J Bacteriol. 1999. PMID: 10515922 Free PMC article.
-
Sodium-driven motor of the polar flagellum in marine bacteria Vibrio.Genes Cells. 2011 Oct;16(10):985-99. doi: 10.1111/j.1365-2443.2011.01545.x. Epub 2011 Sep 5. Genes Cells. 2011. PMID: 21895888 Review.
-
The Periplasmic Domain of the Ion-Conducting Stator of Bacterial Flagella Regulates Force Generation.Front Microbiol. 2022 Apr 27;13:869187. doi: 10.3389/fmicb.2022.869187. eCollection 2022. Front Microbiol. 2022. PMID: 35572622 Free PMC article. Review.
Cited by
-
Gate-controlled proton diffusion and protonation-induced ratchet motion in the stator of the bacterial flagellar motor.Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):7737-42. doi: 10.1073/pnas.1502991112. Epub 2015 Jun 8. Proc Natl Acad Sci U S A. 2015. PMID: 26056313 Free PMC article.
-
FliL association with flagellar stator in the sodium-driven Vibrio motor characterized by the fluorescent microscopy.Sci Rep. 2018 Jul 24;8(1):11172. doi: 10.1038/s41598-018-29447-x. Sci Rep. 2018. PMID: 30042401 Free PMC article.
-
Essential ion binding residues for Na+ flow in stator complex of the Vibrio flagellar motor.Sci Rep. 2019 Aug 2;9(1):11216. doi: 10.1038/s41598-019-46038-6. Sci Rep. 2019. PMID: 31375690 Free PMC article.
-
Ancestral Sequence Reconstructions of MotB Are Proton-Motile and Require MotA for Motility.Front Microbiol. 2020 Dec 23;11:625837. doi: 10.3389/fmicb.2020.625837. eCollection 2020. Front Microbiol. 2020. PMID: 33424826 Free PMC article.
-
The rapid evolution of flagellar ion selectivity in experimental populations of E. coli.Sci Adv. 2022 Nov 25;8(47):eabq2492. doi: 10.1126/sciadv.abq2492. Epub 2022 Nov 23. Sci Adv. 2022. PMID: 36417540 Free PMC article.
References
-
- Yorimitsu T, Homma M. Na(+)-driven flagellar motor of Vibrio. Biochim Biophys Acta. 2001;1505:82–93. - PubMed
-
- Berg HC. The rotary motor of bacterial flagella. Annu Rev Biochem. 2003;72:19–54. - PubMed
-
- Blair DF. Flagellar movement driven by proton translocation. FEBS Lett. 2003;545:86–95. - PubMed
-
- Sato K, Homma M. Functional reconstitution of the Na(+)-driven polar flagellar motor component of vibrio alginolyticus. J Biol Chem. 2000;275:5718–5722. - PubMed
LinkOut - more resources
Full Text Sources
