T Cells Exacerbate Lyme Borreliosis in TLR2-Deficient Mice
- PMID: 27857714
- PMCID: PMC5093308
- DOI: 10.3389/fimmu.2016.00468
T Cells Exacerbate Lyme Borreliosis in TLR2-Deficient Mice
Abstract
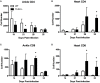
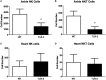
Infection of humans with the spirochete, Borrelia burgdorferi, causes Lyme borreliosis and can lead to clinical manifestations such as arthritis, carditis, and neurological conditions. Experimental infection of mice recapitulates many of these symptoms and serves as a model system for the investigation of disease pathogenesis and immunity. Innate immunity is known to drive the development of Lyme arthritis and carditis, but the mechanisms driving this response remain unclear. Innate immune cells recognize B. burgdorferi surface lipoproteins primarily via toll-like receptor (TLR)2; however, previous work has demonstrated TLR2-/- mice had exacerbated disease and increased bacterial burden. We demonstrate increased CD4 and CD8 T cell infiltrates in B. burgdorferi-infected joints and hearts of C3H TLR2-/- mice. In vivo depletion of either CD4 or CD8 T cells reduced Borrelia-induced joint swelling and lowered tissue spirochete burden, whereas depletion of CD8 T cells alone reduced disease severity scores. Exacerbation of Lyme arthritis correlated with increased production of CXCL9 by synoviocytes, and this was reduced with CD8 T cell depletion. These results demonstrate T cells can exacerbate Lyme disease pathogenesis and prolong disease resolution possibly through dysregulation of inflammatory responses and inhibition of bacterial clearance.
Keywords: Borrelia burgdorferi; Lyme disease; T cells; arthritis; mouse; toll-like receptor 2.
Figures
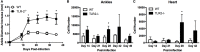





Similar articles
-
Recruitment of macrophages and polymorphonuclear leukocytes in Lyme carditis.Infect Immun. 2007 Feb;75(2):613-20. doi: 10.1128/IAI.00685-06. Epub 2006 Nov 13. Infect Immun. 2007. PMID: 17101663 Free PMC article.
-
CD4+ T helper 1 cells facilitate regression of murine Lyme carditis.Infect Immun. 2001 Sep;69(9):5264-9. doi: 10.1128/IAI.69.9.5264-5269.2001. Infect Immun. 2001. PMID: 11500394 Free PMC article.
-
Lyme borreliosis in transgenic mice tolerant to Borrelia burgdorferi OspA or B.J Clin Invest. 1995 Oct;96(4):1706-14. doi: 10.1172/JCI118215. J Clin Invest. 1995. PMID: 7560061 Free PMC article.
-
Infectious arthritis and immune dysregulation: lessons from Lyme disease.Curr Opin Rheumatol. 2010 Jul;22(4):451-5. doi: 10.1097/BOR.0b013e328338f73f. Curr Opin Rheumatol. 2010. PMID: 20375899 Review.
-
Mechanisms of Borrelia burgdorferi internalization and intracellular innate immune signaling.Front Cell Infect Microbiol. 2014 Dec 15;4:175. doi: 10.3389/fcimb.2014.00175. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25566512 Free PMC article. Review.
Cited by
-
Human Fcγ-receptor IIb modulates pathogen-specific versus self-reactive antibody responses in lyme arthritis.Elife. 2020 Jul 2;9:e55319. doi: 10.7554/eLife.55319. Elife. 2020. PMID: 32613944 Free PMC article.
-
Spirochetal Lipoproteins and Immune Evasion.Front Immunol. 2017 Mar 29;8:364. doi: 10.3389/fimmu.2017.00364. eCollection 2017. Front Immunol. 2017. PMID: 28424696 Free PMC article. Review.
-
CD4 T cell responses in persistent Borrelia burgdorferi infection.Curr Opin Immunol. 2022 Aug;77:102187. doi: 10.1016/j.coi.2022.102187. Epub 2022 May 9. Curr Opin Immunol. 2022. PMID: 35550259 Free PMC article. Review.
-
Protozoan Parasite Babesia microti Subverts Adaptive Immunity and Enhances Lyme Disease Severity.Front Microbiol. 2019 Jul 10;10:1596. doi: 10.3389/fmicb.2019.01596. eCollection 2019. Front Microbiol. 2019. PMID: 31354683 Free PMC article.
-
A murine model of Lyme disease demonstrates that Borrelia burgdorferi colonizes the dura mater and induces inflammation in the central nervous system.PLoS Pathog. 2021 Feb 1;17(2):e1009256. doi: 10.1371/journal.ppat.1009256. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33524035 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

