Comparative assessment of antimicrobial efficacy of different hand sanitizers: An in vitro study
- PMID: 27857768
- PMCID: PMC5091001
- DOI: 10.4103/1735-3327.192283
Comparative assessment of antimicrobial efficacy of different hand sanitizers: An in vitro study
Abstract
Background: To evaluate the antimicrobial efficacy of four different hand sanitizers against Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli, and Enterococcus faecalis as well as to assess and compare the antimicrobial effectiveness among four different hand sanitizers.
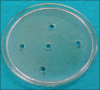
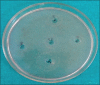
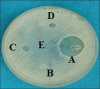
Materials and methods: The present study is an in vitro study to evaluate antimicrobial efficacy of Dettol, Lifebuoy, PureHands, and Sterillium hand sanitizers against clinical isolates of the aforementioned test organisms. The well variant of agar disk diffusion test using Mueller-Hinton agar was used for evaluating the antimicrobial efficacy of hand sanitizers. McFarland 0.5 turbidity standard was taken as reference to adjust the turbidity of bacterial suspensions. Fifty microliters of the hand sanitizer was introduced into each of the 4 wells while the 5th well incorporated with sterile water served as a control. This was done for all the test organisms and plates were incubated in an incubator for 24 h at 37C. After incubation, antimicrobial effectiveness was determined using digital caliper (mm) by measuring the zone of inhibition.
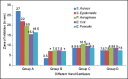
Results: The mean diameters of zones of inhibition (in mm) observed in Group A (Sterillium), Group B (PureHands), Group C (Lifebuoy), and Group D (Dettol) were 22 ± 6, 7.5 ± 0.5, 9.5 ± 1.5, and 8 ± 1, respectively. Maximum inhibition was found with Group A against all the tested organisms. Data were statistically analyzed using analysis of variance, followed by post hoc test for group-wise comparisons. The difference in the values of different sanitizers was statistically significant at P < 0.001.
Conclusion: Sterillium was the most effective hand sanitizer to maintain the hand hygiene.
Keywords: Anti infective agent; hand sanitizers; hygiene; organisms; test.
Figures

References
-
- Hassan AO, Hassan RO, Muhibi MA, Adebimpe WO. A survey of Enterobacteriaceae in hospital and community acquired infections among adults in a tertiary health institution in Southwestern Nigeria. Afr J Microbiol Res. 2012;6:5162–7.
-
- Mondal S, Kolhapure SA. Evaluation of the antimicrobial efficacy and safety of pure hands herbal hand sanitizer in hand hygiene and on inanimate objects. Antiseptic. 2004;101:55–7.
-
- Pratt RJ, Pellowe C, et al. The epic project: Developing national evidence-based guidelines for preventing healthcare associated infections. Phase 1: Guidelines for preventing hospital-acquired infections. Department of Health (England) J Hosp Infect. 2001;47(Suppl 1):S3–82. - PubMed
-
- Centers for Disease Control and Prevention. Guideline for Hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. MMWR. 2002;51:1–56.
-
- World Health Organization. WHO/IER/PSP/2009/01. Geneva, Switzerland: World Health Organization; 2009. WHO Guidelines in Hand Hygiene in Health Care.
LinkOut - more resources
Full Text Sources
Other Literature Sources
