STAT3 Inhibition as a Therapeutic Strategy for Chordoma
- PMID: 27857879
- PMCID: PMC5112170
- DOI: 10.1055/s-0036-1584198
STAT3 Inhibition as a Therapeutic Strategy for Chordoma
Abstract
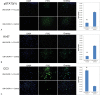
Objective Signal transducer and activator of transcription (STAT) proteins regulate key cellular fate decisions including proliferation and apoptosis. STAT3 overexpression induces tumor growth in multiple neoplasms. STAT3 is constitutively activated in chordoma, a tumor with a high recurrence rate despite maximal surgical and radiation treatment. We hypothesized that a novel small molecule inhibitor of STAT3 (FLLL32) would induce significant cytotoxicity in sacral and clival chordoma cells. Methods Sacral (UCh1) and clival (UM-CHOR-1) chordoma cell lines were grown in culture (the latter derived from primary tumor explants). FLLL32 dosing parameters were optimized using cell viability assays. Antitumor potential of FLLL32 was assessed using clonal proliferation assays. Potential mechanisms underlying observed cytotoxicity were examined using immunofluorescence assays. Results FLLL32 induced significant cytotoxicity in UCh1 and UM-CHOR-1 chordoma cells, essentially eliminating all viable cells, correlating with observed downregulation in activated, phosphorylated STAT3 upon administration of FLLL32. Mechanisms underlying the observed cytotoxicity included increased apoptosis and reduced cellular proliferation through inhibition of mitosis. Conclusion As a monotherapy, FLLL32 induces potent tumor kill in vitro in chordoma cell lines derived from skull base and sacrum. This effect is mediated through inhibition of STAT3 phosphorylation, increased susceptibility to apoptosis, and suppression of cell proliferation.
Keywords: FLLL32; STAT3; chordoma; sacrum; skull base.
Conflict of interest statement
Figures





References
-
- Walcott B P, Nahed B V, Mohyeldin A, Coumans J V, Kahle K T, Ferreira M J. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–e76. - PubMed
-
- McMaster M L, Goldstein A M, Bromley C M, Ishibe N, Parry D M. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12(1):1–11. - PubMed
-
- Williams B J, Raper D M, Godbout E. et al.Diagnosis and treatment of chordoma. J Natl Compr Canc Netw. 2013;11(6):726–731. - PubMed
-
- Stacchiotti S, Casali P G. Systemic therapy options for unresectable and metastatic chordomas. Curr Oncol Rep. 2011;13(4):323–330. - PubMed
-
- Di Maio S, Temkin N, Ramanathan D, Sekhar L N. Current comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studies. J Neurosurg. 2011;115(6):1094–1105. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

