Functional analysis of RYR1 variants linked to malignant hyperthermia
- PMID: 27857962
- PMCID: PMC4964997
- DOI: 10.1080/23328940.2016.1153360
Functional analysis of RYR1 variants linked to malignant hyperthermia
Abstract
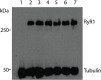
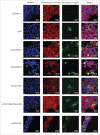
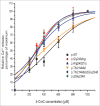
Malignant hyperthermia manifests as a rapid and sustained rise in temperature in response to pharmacological triggering agents, e.g. inhalational anesthetics and the muscle relaxant suxamethonium. Other clinical signs include an increase in end-tidal CO2, increased O2 consumption, as well as tachycardia, and if untreated a malignant hyperthermia episode can result in death. The metabolic changes are caused by dysregulation of skeletal muscle Ca2+ homeostasis, resulting from a defective ryanodine receptor Ca2+ channel, which resides in the sarcoplasmic reticulum and controls the flux of Ca2+ ions from intracellular stores to the cytoplasm. Most genetic variants associated with susceptibility to malignant hyperthermia occur in the RYR1 gene encoding the ryanodine receptor type 1. While malignant hyperthermia susceptibility can be diagnosed by in vitro contracture testing of skeletal muscle biopsy tissue, it is advantageous to use DNA testing. Currently only 35 of over 400 potential variants in RYR1 have been classed as functionally causative of malignant hyperthermia and thus can be used for DNA diagnostic tests. Here we describe functional analysis of 2 RYR1 variants (c. 7042_7044delCAG, p.ΔGlu2348 and c.641C>T, p.Thr214Met) that occur in the same malignant hyperthermia susceptible family. The p.Glu2348 deletion, causes hypersensitivity to ryanodine receptor agonists using in vitro analysis of cloned human RYR1 cDNA expressed in HEK293T cells, while the Thr214Met substitution, does not appear to significantly alter sensitivity to agonist in the same system. We suggest that the c. 7042_7044delCAG, p.ΔGlu2348 RYR1 variant could be added to the list of diagnostic mutations for susceptibility to malignant hyperthermia.
Keywords: anesthesia; calcium channel; malignant hyperthermia; ryanodine receptor; skeletal muscle.
Figures

References
-
- MacLennan DH, Chen SR. The role of the calcium release channel of skeletal muscle sarcoplasmic reticulum in malignant hyperthermia. Ann N Y Acad Sci 1993; 707:294-304; PMID:9137560; http://dx.doi.org/10.1111/j.1749-6632.1993.tb38060.x - DOI - PubMed
-
- Hopkins PM. Malignant hyperthermia: pharmacology of triggering. Br J Anaesth 2011; 107:48-56; PMID:21624965; http://dx.doi.org/10.1093/bja/aer132 - DOI - PubMed
-
- Vukcevic M, Broman M, Islander G, Bodelsson M, Ranklev-Twetman E, Muller CR, Treves S. Functional properties of RYR1 mutations identified in Swedish patients with malignant hyperthermia and central core disease. Anesth Analg 2010:111:185-90; PMID:20142353 - PubMed
-
- Yang T, Esteve E, Pessah IN, Molinski TF, Allen PD, Lopez JR. Elevated resting [Ca2+]i in myotubes expressing malignant hyperthermia RyR1 cDNAs is partially restored by modulation of passive calcium leak from the SR. Am J Physiol Cell Physiol 2007:292:C1591-8 Epub 2006 Dec 20; PMID:17182726; http://dx.doi.org/10.1152/ajpcell.00133.2006 - DOI - PubMed
-
- Eltit JM, Bannister RA, Moua O, Altamirano F, Hopkins PM, Pessah IN, Molinski TF, Lopez JR, Beam KG, Allen PD. Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc Natl Acad Sci U S A 2012; 109:7923-8; PMID:22547813; http://dx.doi.org/10.1073/pnas.1119207109 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
