Development and evaluation of a standardized ELISA for the determination of autoantibodies against cN-1A (Mup44, NT5C1A) in sporadic inclusion body myositis
- PMID: 27858337
- PMCID: PMC5114199
- DOI: 10.1007/s13317-016-0088-8
Development and evaluation of a standardized ELISA for the determination of autoantibodies against cN-1A (Mup44, NT5C1A) in sporadic inclusion body myositis
Abstract
Purpose: Sporadic inclusion body myositis (sIBM) is an autoimmune degenerative disease of the muscle, with inflammatory infiltrates and inclusion vacuoles. Its pathogenesis is not fully understood and the diagnosis is hampered by its imprecise characteristics, at times indistinguishable from other idiopathic inflammatory myopathies such as polymyositis and dermatomyositis. The diagnosis may be assisted by the detection of autoantibodies targeting Mup44, a skeletal muscle antigen identified as cytosolic 5'-nucleotidase 1A (cN-1A, NT5C1A). A novel standardized anti-cN-1A IgG ELISA was developed and its diagnostic performance was evaluated by two reference laboratories.
Methods: Recombinant human full-length cN-1A was expressed and purified, and subsequently utilized to set up a standardized ELISA. To evaluate the novel assay, laboratory A examined sera from North American patients with clinically and pathologically diagnosed definite sIBM (n = 17), suspected sIBM (n = 14), myositis controls (n = 110), non-myositis autoimmune controls (n = 93) and healthy subjects (n = 52). Laboratory B analyzed a Dutch cohort of definite sIBM patients (n = 51) and healthy controls (n = 202).
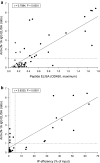
Results: Anti-cN-1A reactivity was most frequent in definite sIBM (39.2-47.1%), but absent in biopsy-proven classic polymyositis or dermatomyositis. Overall diagnostic sensitivity and specificity amounted to 35.5 and 96.1% (laboratory A) and 39.2 and 96.5% (laboratory B).
Conclusions: Anti-cN-1A autoantibodies were detected by ELISA with moderate sensitivity, but high specificity for sIBM and may therefore help diagnose this infrequent and difficult-to-diagnose myopathy. The novel anti-cN-1A IgG ELISA can improve and accelerate the diagnosis of sIBM using sera where muscle biopsy is delayed or unfeasible.
Keywords: Anti-Mup44; Anti-cN-1A; Autoantibodies; Autoimmunity; ELISA; Myopathy; Sporadic inclusion body myositis.
Conflict of interest statement
Compliance with ethical standardsConflict of interestDmitry Karayev, Guo Shen, Allan L. Metzger, Robert I. Morris, Eugene Karayev, Yvonne Lam and Richard M. Kazdan are employees of RDL Reference Laboratory Inc. Allan L. Metzger and Robert I. Morris are shareholders of RDL Reference Laboratory Inc. Ger J. M. Pruijn is inventor of a patent (EP20120740236) licensed to Euroimmun AG. Sabine L. Kramp, Sandra Saschenbrecker, Cornelia Dähnrich and Wolfgang Schlumberger are employees of Euroimmun AG. Wolfgang Schlumberger is a board member of Euroimmun AG. Cornelia Dähnrich and Wolfgang Schlumberger are shareholders of Euroimmun AG. Euroimmun AG is a diagnostic manufacturer.Ethical approvalAll study procedures were in accordance with the ethical standards of the institutional research committees and with the Helsinki Declaration.Human and animal rightsAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.Informed consentFor this type of study formal consent is not required.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

