Reactivation of seizure-related changes to interictal spike shape and synchrony during postseizure sleep in patients
- PMID: 27859029
- PMCID: PMC5358812
- DOI: 10.1111/epi.13614
Reactivation of seizure-related changes to interictal spike shape and synchrony during postseizure sleep in patients
Abstract
Objective: Local field potentials (LFPs) arise from synchronous activation of millions of neurons, producing seemingly consistent waveform shapes and relative synchrony across electrodes. Interictal spikes (IISs) are LFPs associated with epilepsy that are commonly used to guide surgical resection. Recently, changes in neuronal firing patterns observed in the minutes preceding seizure onset were found to be reactivated during postseizure sleep, a process called seizure-related consolidation (SRC), due to similarities with learning-related consolidation. Because IISs arise from summed neural activity, we hypothesized that changes in IIS shape and relative synchrony would be observed in the minutes preceding seizure onset and would be reactivated preferentially during postseizure slow-wave sleep (SWS).
Methods: Scalp and intracranial recordings were obtained continuously across multiple days from clinical macroelectrodes implanted in patients undergoing treatment for intractable epilepsy. Data from scalp electrodes were used to stage sleep. Data from intracranial electrodes were used to detect IISs using a previously established algorithm. Partial correlations were computed for sleep and wake periods before and after seizures as a function of correlations observed in the minutes preceding seizures. Magnetic resonance imaging (MRI) and computed tomography (CT) scans were co-registered with electroencephalography (EEG) to determine the location of the seizure-onset zone (SOZ).
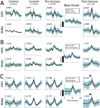
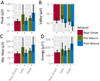
Results: Changes in IIS shape and relative synchrony were observed on a subset of macroelectrodes minutes before seizure onset, and these changes were reactivated preferentially during postseizure SWS. Changes in synchrony were greatest for pairs of electrodes where at least one electrode was located in the SOZ.
Significance: These data suggest preseizure changes in neural activity and their subsequent reactivation occur across a broad spatiotemporal scale: from single neurons to LFPs, both within and outside the SOZ. The preferential reactivation of seizure-related changes in IISs during postseizure SWS adds to a growing body of literature suggesting that pathologic neural processes may utilize physiologic mechanisms of synaptic plasticity.
Keywords: EEG; Interictal spike; Neural plasticity; Seizure.
Wiley Periodicals, Inc. © 2016 International League Against Epilepsy.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

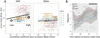


References
-
- Skaggs WE, McNaughton BL, Wilson MA, et al. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. - PubMed
-
- Zhou J-L, Lenck-Santini P-P, Zhao Q, et al. Effect of interictal spikes on single-cell firing patterns in the hippocampus. Epilepsia. 2007;48:720–731. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

