Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models
- PMID: 27859259
- PMCID: PMC5248628
- DOI: 10.1002/path.4845
Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models
Abstract
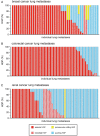
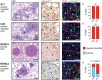
Anti-angiogenic therapies have shown limited efficacy in the clinical management of metastatic disease, including lung metastases. Moreover, the mechanisms via which tumours resist anti-angiogenic therapies are poorly understood. Importantly, rather than utilizing angiogenesis, some metastases may instead incorporate pre-existing vessels from surrounding tissue (vessel co-option). As anti-angiogenic therapies were designed to target only new blood vessel growth, vessel co-option has been proposed as a mechanism that could drive resistance to anti-angiogenic therapy. However, vessel co-option has not been extensively studied in lung metastases, and its potential to mediate resistance to anti-angiogenic therapy in lung metastases is not established. Here, we examined the mechanism of tumour vascularization in 164 human lung metastasis specimens (composed of breast, colorectal and renal cancer lung metastasis cases). We identified four distinct histopathological growth patterns (HGPs) of lung metastasis (alveolar, interstitial, perivascular cuffing, and pushing), each of which vascularized via a different mechanism. In the alveolar HGP, cancer cells invaded the alveolar air spaces, facilitating the co-option of alveolar capillaries. In the interstitial HGP, cancer cells invaded the alveolar walls to co-opt alveolar capillaries. In the perivascular cuffing HGP, cancer cells grew by co-opting larger vessels of the lung. Only in the pushing HGP did the tumours vascularize by angiogenesis. Importantly, vessel co-option occurred with high frequency, being present in >80% of the cases examined. Moreover, we provide evidence that vessel co-option mediates resistance to the anti-angiogenic drug sunitinib in preclinical lung metastasis models. Assuming that our interpretation of the data is correct, we conclude that vessel co-option in lung metastases occurs through at least three distinct mechanisms, that vessel co-option occurs frequently in lung metastases, and that vessel co-option could mediate resistance to anti-angiogenic therapy in lung metastases. Novel therapies designed to target both angiogenesis and vessel co-option are therefore warranted. © 2016 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: angiogenesis; anti-angiogenic therapy; drug resistance; lung metastasis; sunitinib; vessel co-option.
© 2016 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

