Post-translational cleavage of Hv1 in human sperm tunes pH- and voltage-dependent gating
- PMID: 27859356
- PMCID: PMC5330862
- DOI: 10.1113/JP273189
Post-translational cleavage of Hv1 in human sperm tunes pH- and voltage-dependent gating
Abstract
Key points: In human sperm, proton flux across the membrane is controlled by the voltage-gated proton channel Hv1. We show that sperm harbour both Hv1 and an N-terminally cleaved isoform termed Hv1Sper. The pH-control of Hv1Sper and Hv1 is distinctively different. Hv1Sper and Hv1 can form heterodimers that combine features of both constituents. Cleavage and heterodimerization of Hv1 might represent an adaptation to the specific requirements of pH control in sperm.
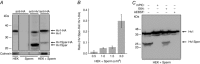
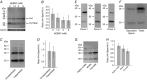
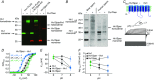
Abstract: In human sperm, the voltage-gated proton channel Hv1 controls the flux of protons across the flagellar membrane. Here, we show that sperm harbour Hv1 and a shorter isoform, termed Hv1Sper. Hv1Sper is generated from Hv1 by removal of 68 amino acids from the N-terminus by post-translational proteolytic cleavage. The pH-dependent gating of the channel isoforms is distinctly different. In both Hv1 and Hv1Sper, the conductance-voltage relationship is determined by the pH difference across the membrane (∆pH). However, simultaneous changes in intracellular and extracellular pH that leave ΔpH constant strongly shift the activation curve of Hv1Sper but not that of Hv1, demonstrating that cleavage of the N-terminus tunes pH sensing in Hv1. Moreover, we show that Hv1 and Hv1Sper assemble as heterodimers that combine features of both constituents. We suggest that cleavage and heterodimerization of Hv1 represents an adaptation to the specific requirements of pH control in sperm.
Keywords: electrophysiology; ion channels; sperm; voltage-gated channels.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures



References
-
- Austin CR (1951). Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B 4, 581–596. - PubMed
-
- Batra‐Safferling R, Abarca‐Heidemann K, Körschen HG, Tziatzios C, Stoldt M, Budyak I, Willbold D, Schwalbe H, Klein‐Seetharaman J & Kaupp UB (2006). Glutamic acid‐rich proteins of rod photoreceptors are natively unfolded. J Biol Chem 281, 1449–1460. - PubMed
-
- Bönigk W, Loogen A, Seifert R, Kashikar N, Klemm C, Krause E, Hagen V, Kremmer E, Strünker T & Kaupp UB (2009). An atypical CNG channel activated by a single cGMP molecule controls sperm chemotaxis. Sci Signal 2, ra68. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

