Sildenafil attenuates hypoxic pulmonary remodelling by inhibiting bone marrow progenitor cells
- PMID: 27860185
- PMCID: PMC5387166
- DOI: 10.1111/jcmm.13026
Sildenafil attenuates hypoxic pulmonary remodelling by inhibiting bone marrow progenitor cells
Abstract
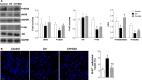
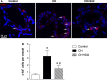
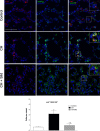
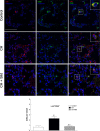
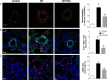
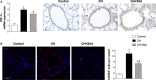
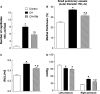
The recruitment of bone marrow (BM)-derived progenitor cells to the lung is related to pulmonary remodelling and the pathogenesis of pulmonary hypertension (PH). Although sildenafil is a known target in PH treatment, the underlying molecular mechanism is still elusive. To test the hypothesis that the therapeutic effect of sildenafil is linked to the reduced recruitment of BM-derived progenitor cells, we induced pulmonary remodelling in rats by two-week exposure to chronic hypoxia (CH, 10% oxygen), a trigger of BM-derived progenitor cells. Rats were treated with either placebo (saline) or sildenafil (1.4 mg/kg/day ip) during CH. Control rats were kept in room air (21% oxygen) with no treatment. As expected, sildenafil attenuated the CH-induced increase in right ventricular systolic pressure and right ventricular hypertrophy. However, sildenafil suppressed the CH-induced increase in c-kit+ cells in the adventitia of pulmonary arteries. Moreover, sildenafil reduced the number of c-kit+ cells that colocalize with tyrosine kinase receptor 2 (VEGF-R2) and CD68 (a marker for macrophages), indicating a positive effect on moderating hypoxia-induced smooth muscle cell proliferation and inflammation without affecting the pulmonary levels of hypoxia-inducible factor (HIF)-1α. Furthermore, sildenafil depressed the number of CXCR4+ cells. Collectively, these findings indicate that the improvement in pulmonary haemodynamic by sildenafil is linked to decreased recruitment of BM-derived c-kit+ cells in the pulmonary tissue. The attenuation of the recruitment of BM-derived c-kit+ cells by sildenafil may provide novel therapeutic insights into the control of pulmonary remodelling.
Keywords: CXCR4 receptor; bone marrow progenitor cells; c-kit cells; chronic hypoxia; pulmonary hypertension; sildenafil.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
Similar articles
-
Statin ameliorates hypoxia-induced pulmonary hypertension associated with down-regulated stromal cell-derived factor-1.Cardiovasc Res. 2009 Jan 1;81(1):226-34. doi: 10.1093/cvr/cvn244. Epub 2008 Sep 8. Cardiovasc Res. 2009. PMID: 18779230
-
Targeting of c-kit+ haematopoietic progenitor cells prevents hypoxic pulmonary hypertension.Eur Respir J. 2011 Jun;37(6):1392-9. doi: 10.1183/09031936.00045710. Epub 2010 Sep 30. Eur Respir J. 2011. PMID: 20884740
-
Contribution of CXCR4(+)/PDGFRbeta(+) progenitor cells in hypoxic alveolar arterioles muscularization: role of myocardin.Cardiovasc Res. 2010 Sep 1;87(4):740-50. doi: 10.1093/cvr/cvq147. Epub 2010 May 18. Cardiovasc Res. 2010. PMID: 20484220
-
C-Kit/c-Kit ligand interaction of bone marrow endothelial progenitor cells is influenced in a cigarette smoke extract-induced emphysema model.Exp Lung Res. 2013 Aug;39(6):258-67. doi: 10.3109/01902148.2013.802828. Epub 2013 Jun 20. Exp Lung Res. 2013. PMID: 23786491
-
A reappraisal of the role of circulating (progenitor) cells in the pathobiology of diabetic complications.Diabetologia. 2014 Jan;57(1):4-15. doi: 10.1007/s00125-013-3087-6. Epub 2013 Oct 31. Diabetologia. 2014. PMID: 24173366 Review.
Cited by
-
Silibinin efficacy in a rat model of pulmonary arterial hypertension using monocrotaline and chronic hypoxia.Respir Res. 2019 Apr 25;20(1):79. doi: 10.1186/s12931-019-1041-y. Respir Res. 2019. PMID: 31023308 Free PMC article.
-
Origin and production of inflammatory perivascular macrophages in pulmonary hypertension.Cytokine. 2017 Dec;100:11-15. doi: 10.1016/j.cyto.2017.08.015. Epub 2017 Aug 30. Cytokine. 2017. PMID: 28855075 Free PMC article. Review.
-
High expression of CXCR4 and stem cell markers in a monocrotaline and chronic hypoxia-induced rat model of pulmonary arterial hypertension.Exp Ther Med. 2018 Jun;15(6):4615-4622. doi: 10.3892/etm.2018.6027. Epub 2018 Apr 3. Exp Ther Med. 2018. PMID: 29805477 Free PMC article.
-
Nitric Oxide-cGMP Pathway Modulation in an Experimental Model of Hypoxic Pulmonary Hypertension.J Cardiovasc Pharmacol Ther. 2021 Nov;26(6):665-676. doi: 10.1177/10742484211014162. Epub 2021 May 8. J Cardiovasc Pharmacol Ther. 2021. PMID: 33969747 Free PMC article.
-
The Oxygen Cascade from Atmosphere to Mitochondria as a Tool to Understand the (Mal)adaptation to Hypoxia.Int J Mol Sci. 2023 Feb 12;24(4):3670. doi: 10.3390/ijms24043670. Int J Mol Sci. 2023. PMID: 36835089 Free PMC article. Review.
References
-
- McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006; 114: 1417–31. - PubMed
-
- Nickel N, Golpon H, Greer M, et al The prognostic impact of follow‐up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2012; 39: 589–96. - PubMed
-
- Davie NJ, Crossno JT Jr, Frid MG, et al Hypoxia‐induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol. 2004; 286: L668–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

