Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies
- PMID: 27860216
- PMCID: PMC5592089
- DOI: 10.1002/ajmg.c.31531
Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies
Abstract
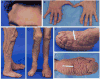
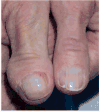
The phosphatidylinositol-3-kinase (PI3K)/AKT/mTOR signaling pathway plays an essential role in regulation of normal cell growth, metabolism, and survival. Somatic activating mutations in the PI3K/AKT/mTOR pathway are among the most common mutations identified in cancer, and have been shown to cause a spectrum of overgrowth syndromes including PIK3CA-Related Overgrowth Spectrum, Proteus syndrome, and brain overgrowth conditions. Clinical findings in these disorders may be isolated or multiple, including sporadic or mosaic overgrowth (adipose, skeletal, muscle, brain, vascular, or lymphatic), and skin abnormalities (including epidermal nevi, hyper-, and hypopigmented lesions), and have the potential risk of tumorigenesis. Key negative regulators of the PI3K-AKT signaling pathway include PTEN and TSC1/TSC2 and germline loss-of function mutations of these genes are established to cause PTEN Hamartoma Tumor Syndrome and Tuberous Sclerosis Complex. Mosaic forms of these conditions lead to increased activation of PI3K and mTOR at affected sites and there is phenotypic overlap between these conditions. All are associated with significant morbidity with limited options for treatment other than symptomatic therapies and surgeries. As dysregulation of the PI3K/AKT/mTOR pathway has been implicated in cancer, several small molecule inhibitors targeting different components of the PI3K/AKT/mTOR signaling pathway are under clinical investigation. The development of these therapies brings closer the prospect of targeting treatment for somatic PI3K/AKT/mTOR-related overgrowth syndromes. This review describes the clinical findings, gene function and pathogenesis of these mosaic overgrowth syndromes, and presents existing and future treatment strategies to reduce or prevent associated complications of these disorders. © 2016 Wiley Periodicals, Inc.
Keywords: PI3K/AKT/mTOR pathway; segmental overgrowth; somatic mosaicism; therapy.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

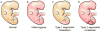



References
-
- Alomari AI, Burrows PE, Lee EY, Hedequist DJ, Mulliken JB, Fishman SJ. CLOVES syndrome with thoracic and central phlebectasia:Increased risk of pulmonary embolism. J Thorac Cardiovasc Surg. 2010;140:459–466. - PubMed
-
- Arch EM, Goodman BK, Van Wesep RA, Liaw D, Clarke K, Parsons R, McKusick VA, Geraghty MT. Deletion of PTEN in a patient with Bannayan-Riley-Ruvalcaba syndrome suggests allelism with Cowden disease. Am J Med Genet A. 1997;71:489–493. - PubMed
-
- Assefa G, Alemie B. Tuberous sclerosis complex (TSC) and Klippel-Trenaunay-Weber (KTW) syndromes association of two complete phakomatoses in a single individual. Ethiop Med J. 2010;48:315–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

