Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology
- PMID: 27860287
- PMCID: PMC5226885
- DOI: 10.1111/febs.13963
Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology
Abstract
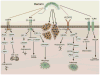
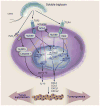
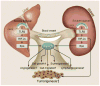
Inflammation and autophagy have emerged as prominent issues in the context of proteoglycan signaling. In particular, two small, leucine-rich proteoglycans, biglycan and decorin, play pivotal roles in the regulation of these vital cellular pathways and, as such, are intrinsically involved in cancer initiation and progression. In this minireview, we will address novel functions of biglycan and decorin in inflammation and autophagy, and analyze new emerging signaling events triggered by these proteoglycans, which directly or indirectly modulate these processes. We will critically discuss the dual role of proteoglycan-driven inflammation and autophagy in tumor biology, and delineate the potential mechanisms through which soluble extracellular matrix constituents affect the microenvironment associated with inflammatory and neoplastic diseases.
Keywords: Toll-like receptors; biglycan; decorin; microtubule-associated protein light chain 3; receptor tyrosine kinase; small leucine-rich proteoglycans.
© 2016 Federation of European Biochemical Societies.
Figures
References
-
- Afratis N, Multhaupt HAB, Nikitovic D, Theocharis AD, Couchman JR, Karamanos NK. Syndecans: key regulators of cell signaling and biological functions. FEBS J. 2016 In press. - PubMed
-
- Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage H, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Rep Reg. 2006;14:443–452. - PubMed
-
- Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA, Iozzo RV. Decorin binds near the C terminus of type I collagen. J Biol Chem. 2000;275:21801–21804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

