Secondary primary malignancies during the lenalidomide-dexamethasone regimen in relapsed/refractory multiple myeloma patients
- PMID: 27860411
- PMCID: PMC5269689
- DOI: 10.1002/cam4.799
Secondary primary malignancies during the lenalidomide-dexamethasone regimen in relapsed/refractory multiple myeloma patients
Abstract
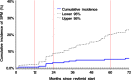
Lenalidomide in combination with dexamethasone (Len-dex) represents a highly effective treatment in relapsed/refractory multiple myeloma (RRMM) patients. However, an increased risk of secondary primary malignancies (SPMs), including myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML) has been described in patients receiving lenalidomide. In order to assess the incidence and features of this complication, we reviewed 195 patients with RRMM treated with Len-dex at our institution. The median follow-up time from diagnosis of MM was 73 months (10-234 months) and from initiation of Len-dex was 19 months (1-104 months). The median duration of Len-dex for all patients was 7.8 months (range 1-90 months). The incidence rate (IR) for all SPMs from start of Len-dex was 2.37 per 100 patient-years, which reflected an IR of 1.29 for MDS/AML and 1.08 for nonhematologic malignancies (NHM). MDS was the most common SPM noted. The cumulative IR of SPM at 5 years was 1.54% from the time of MM diagnosis and 5.24% from starting Len-dex. Multivariable cumulative incidence of SPM analysis identified older age (P = 0.005) and prior number of regimens (P = 0.026) as adverse risk factors. We found more concomitant G-CSF use (P = 0.029) in patients with MDS/AML, however, causal association is not clear. The progression-free survival after Len-dex was the longest for patients in MDS/AML group, and the 5-year overall survival did not differ among groups. Although the rate of SPM was relatively low with Len-dex, concomitant G-CSF should be used judiciously and patients receiving this regimen should be observed for the development of this complication.
Keywords: AML; MDS; lenalidomide; relapsed/refractory multiple myeloma; secondary malignancies.
© 2016 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures


References
-
- Fenk, R. , Neubauer F., Bruns I., et al. 2012. Secondary primary malignancies in patients with multiple myeloma treated with high‐dose chemotherapy and autologous blood stem cell transplantation. Br. J. Haematol. 156:683–686. - PubMed
-
- Dimopoulos, M. , Spencer A., Attal M., Prince H. M., Harousseau J. L., Dmoszynska A., et al. 2007. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N. Engl. J. Med. 357:2123–2132. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

