Effects of levomilnacipran extended-release on major depressive disorder patients with cognitive impairments: post-hoc analysis of a phase III study
- PMID: 27861191
- PMCID: PMC5265686
- DOI: 10.1097/YIC.0000000000000157
Effects of levomilnacipran extended-release on major depressive disorder patients with cognitive impairments: post-hoc analysis of a phase III study
Abstract
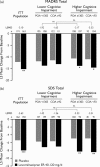
Performance-based cognitive data were collected using the Cognitive Drug Research System in a study of levomilnacipran extended-release (ER) 40-120 mg/day (NCT01034462) in adults with major depressive disorder. These data were analyzed post-hoc to explore the relationship between cognitive measures, depression symptoms (Montgomery-Åsberg Depression Rating Scale, MADRS), and self-reported psychosocial functioning (Sheehan Disability Scale; SDS). Changes from baseline were analyzed in the intent-to-treat population and subgroups with impaired attention, as indicated by baseline Cognitive Drug Research System scores for Power of Attention and Continuity of Attention. Path analyses evaluated the direct and indirect effects of levomilnacipran ER on SDS total score change. Significantly greater improvements were observed for levomilnacipran ER versus placebo for Power of Attention, Continuity of Attention, MADRS, and SDS score changes; the mean differences were larger in the impaired subgroups than in the overall intent-to-treat population. Path analyses showed that the majority of SDS total score improvement (≥50%) was attributable to an indirect treatment effect through MADRS total score change; some direct effect of levomilnacipran ER on SDS total score improvement was also observed. In adults with major depressive disorder, levomilnacipran ER effectively improved measures of depression and cognition, which contributed toward reductions in self-reported functional impairment.
Figures


Similar articles
-
A phase III, double-blind, placebo-controlled, flexible-dose study of levomilnacipran extended-release in patients with major depressive disorder.J Clin Psychopharmacol. 2014 Feb;34(1):47-56. doi: 10.1097/JCP.0000000000000060. J Clin Psychopharmacol. 2014. PMID: 24172209 Free PMC article. Clinical Trial.
-
Effects of levomilnacipran extended-release on motivation/energy and functioning in adults with major depressive disorder.Int Clin Psychopharmacol. 2016 Nov;31(6):332-40. doi: 10.1097/YIC.0000000000000138. Int Clin Psychopharmacol. 2016. PMID: 27455513 Free PMC article. Clinical Trial.
-
The effects of levomilnacipran ER in adult patients with first-episode, highly recurrent, or chronic MDD.J Affect Disord. 2016 Mar 15;193:137-43. doi: 10.1016/j.jad.2015.12.058. Epub 2015 Dec 30. J Affect Disord. 2016. PMID: 26773906 Clinical Trial.
-
Levomilnacipran extended-release: a review of its use in adult patients with major depressive disorder.CNS Drugs. 2014 Nov;28(11):1071-82. doi: 10.1007/s40263-014-0203-1. CNS Drugs. 2014. PMID: 25270036 Review.
-
Levomilnacipran extended release: first global approval.Drugs. 2013 Sep;73(14):1639-45. doi: 10.1007/s40265-013-0116-1. Drugs. 2013. PMID: 24000002 Review.
References
-
- Al-Sukhni M, Maruschak NA, Mcintyre RS. (2015). Vortioxetine: a review of efficacy, safety and tolerability with a focus on cognitive symptoms in major depressive disorder. Expert Opin Drug Saf 14:1291–1304. - PubMed
-
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association.
-
- Baune BT, Renger L. (2014). Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression–a systematic review. Psychiatry Res 219:25–50. - PubMed
-
- Baune BT, Miller R, Mcafoose J, Johnson M, Quirk F, Mitchell D. (2010). The role of cognitive impairment in general functioning in major depression. Psychiatry Res 176:183–189. - PubMed
-
- Biringer A, Rongve A, Lund A. (2009). A review of modern antidepressants’ effects on neurocogntive function. Curr Psychiatry Rev 5:164–174.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

