Effects of target-controlled infusion of high-dose naloxone on pain and hyperalgesia in a human thermal injury model: a study protocol: A randomized, double-blind, placebo-controlled, crossover trial with an enriched design
- PMID: 27861362
- PMCID: PMC5120919
- DOI: 10.1097/MD.0000000000005336
Effects of target-controlled infusion of high-dose naloxone on pain and hyperalgesia in a human thermal injury model: a study protocol: A randomized, double-blind, placebo-controlled, crossover trial with an enriched design
Abstract
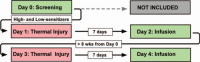
Mu-opioid-receptor antagonists have been extensively studied in experimental research as pharmacological tools uncovering mechanisms of pain modulation by the endogenous opioid system. In rodents, administration of high doses of mu-opioid-receptor antagonists after the resolution of an inflammatory injury has demonstrated reinstatement of nociceptive hypersensitivity indicating unmasking of latent sensitization. In a recent human study, pain hypersensitivity assessed as secondary hyperalgesia area (SHA), was reinstated 7 days after a mild thermal injury, in 4 out of 12 subjects after a naloxone infusion.The aims of the present study are first, to replicate our previous findings in a larger-sized study; second, to examine if high sensitizers (subjects presenting with large SHA after a thermal injury) develop a higher degree of hypersensitivity after naloxone challenge than low sensitizers (subjects presenting with restricted SHA after a thermal injury); and third to examine a dose-response relationship between 3 stable naloxone concentrations controlled by target-controlled infusion, and the unmasking of latent sensitization.Healthy participants (n = 80) underwent a screening day (day 0) with induction of a thermal skin injury (47°C, 420 seconds, 12.5 cm). Assessment of SHA was performed 1 and 2 hours after the injury. Using an enriched design, only participants belonging to the upper quartile of SHA (Q4, high sensitizers; n = 20) and the lower quartile of SHA (Q1, low sensitizers; n = 20) continued the study, comprising 4 consecutive days-days 1 to 4. Thermal skin injuries were repeated on day 1 and day 3, whereas day 2 and day 4 (7 days after day 1 and day 3, respectively) were target-controlled infusion days in which the subjects were randomly allocated to receive either naloxone (3.25 mg/kg, 4 mg/mL) or placebo (normal saline) intravenous. The primary outcome was SHA assessed by weighted-pin instrument (128 mN) 0, 1, 2, and 165 to 169 hours after the thermal injury (day 1-4). The secondary outcomes were pin-prick pain thresholds assessed by weighted-pin instrument (8-512 mN) at primary and secondary hyperalgesia areas (days 1-4).The naloxone-induced unmasking of latent sensitization is an interesting model for exploring the transition from acute to chronic pain. The results from the present study may provide valuable information regarding future research in persistent postsurgical pain states.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures

References
-
- Pielsticker A, Haag G, Zaudig M, et al. Impairment of pain inhibition in chronic tension-type headache. Pain 2005; 118:215–223. - PubMed
-
- Price DD, Staud R, Robinson ME, et al. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 2002; 99:49–59. - PubMed
-
- van Wilgen CP, Keizer D. The sensitization model to explain how chronic pain exists without tissue damage. Pain Manag Nurs 2012; 13:60–65. - PubMed
-
- Campillo A, Cabanero D, Romero A, et al. Delayed postoperative latent pain sensitization revealed by the systemic administration of opioid antagonists in mice. Eur J Pharmacol 2011; 657:89–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

