Cyclooxygenase Isoform Exchange Blocks Brain-Mediated Inflammatory Symptoms
- PMID: 27861574
- PMCID: PMC5115700
- DOI: 10.1371/journal.pone.0166153
Cyclooxygenase Isoform Exchange Blocks Brain-Mediated Inflammatory Symptoms
Abstract
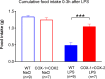
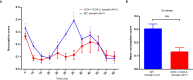
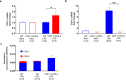
Cyclooxygenase-2 (COX-2) is the main source of inducible prostaglandin E2 production and mediates inflammatory symptoms including fever, loss of appetite and hyperalgesia. COX-1 is dispensable for fever, anorexia and hyperalgesia but is important for several other functions both under basal conditions and during inflammation. The differential functionality of the COX isoforms could be due to differences in the regulatory regions of the genes, leading to different expression patterns, or to differences in the coding sequence, resulting in distinct functional properties of the proteins. To study the molecular underpinnings of the functional differences between the two isoforms in the context of inflammatory symptoms, we used mice in which the coding sequence of COX-2 was replaced by the corresponding sequence of COX-1. In these mice, COX-1 mRNA was induced by inflammation but COX-1 protein expression did not fully mimic inflammation-induced COX-2 expression. Just like mice globally lacking COX-2, these mice showed a complete lack of fever and inflammation-induced anorexia as well as an impaired response to inflammatory pain. However, as previously reported, they displayed close to normal survival rates, which contrasts to the high fetal mortality in COX-2 knockout mice. This shows that the COX activity generated from the hybrid gene was strong enough to allow survival but not strong enough to mediate the inflammatory symptoms studied, making the line an interesting alternative to COX-2 knockouts for the study of inflammation. Our results also show that the functional differences between COX-1 and COX-2 in the context of inflammatory symptoms are not only dependent on the features of the promoter regions. Instead they indicate that there are fundamental differences between the isoforms at translational or posttranslational levels.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The involvement of a cyclooxygenase 1 gene-derived protein in the antinociceptive action of paracetamol in mice.Eur J Pharmacol. 2006 May 24;538(1-3):57-65. doi: 10.1016/j.ejphar.2006.03.061. Epub 2006 Apr 1. Eur J Pharmacol. 2006. PMID: 16674937
-
Cyclooxygenase knockout mice: models for elucidating isoform-specific functions.Biochem Pharmacol. 1999 Oct 15;58(8):1237-46. doi: 10.1016/s0006-2952(99)00158-6. Biochem Pharmacol. 1999. PMID: 10487525
-
COX-2 inhibition attenuates anorexia during systemic inflammation without impairing cytokine production.Am J Physiol Endocrinol Metab. 2002 Mar;282(3):E650-6. doi: 10.1152/ajpendo.00388.2001. Am J Physiol Endocrinol Metab. 2002. PMID: 11832369
-
Cyclooxygenase-deficient mice. A summary of their characteristics and susceptibilities to inflammation and carcinogenesis.Ann N Y Acad Sci. 1999;889:52-61. doi: 10.1111/j.1749-6632.1999.tb08723.x. Ann N Y Acad Sci. 1999. PMID: 10668482 Review.
-
Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors.Inflamm Res. 2000 Aug;49(8):367-92. doi: 10.1007/s000110050605. Inflamm Res. 2000. PMID: 11028754 Review.
Cited by
-
Genomic and lipidomic analyses differentiate the compensatory roles of two COX isoforms during systemic inflammation in mice.J Lipid Res. 2018 Jan;59(1):102-112. doi: 10.1194/jlr.M080028. Epub 2017 Nov 27. J Lipid Res. 2018. PMID: 29180443 Free PMC article.
-
NSP2 Is Important for Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus to Trigger High Fever-Related COX-2-PGE2 Pathway in Pigs.Front Immunol. 2021 Apr 29;12:657071. doi: 10.3389/fimmu.2021.657071. eCollection 2021. Front Immunol. 2021. PMID: 33995374 Free PMC article.
-
Flipping the cyclooxygenase (Ptgs) genes reveals isoform-specific compensatory functions.J Lipid Res. 2018 Jan;59(1):89-101. doi: 10.1194/jlr.M079996. Epub 2017 Nov 27. J Lipid Res. 2018. PMID: 29180445 Free PMC article.
-
PLPP/CIN-mediated NF2 S10 dephosphorylation distinctly regulates kainate-induced seizure susceptibility and neuronal death through PAK1-NF-κB-COX-2-PTGES2 signaling pathway.J Neuroinflammation. 2023 Apr 28;20(1):99. doi: 10.1186/s12974-023-02788-9. J Neuroinflammation. 2023. PMID: 37118736 Free PMC article.
References
-
- Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83(3):473–82. Epub 1995/11/03. . - PubMed
-
- Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83(3):483–92. Epub 1995/11/03. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

