Cellular Basis of Pineal Gland Development: Emerging Role of Microglia as Phenotype Regulator
- PMID: 27861587
- PMCID: PMC5115862
- DOI: 10.1371/journal.pone.0167063
Cellular Basis of Pineal Gland Development: Emerging Role of Microglia as Phenotype Regulator
Abstract
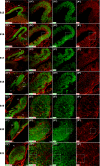
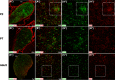
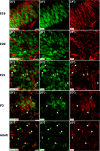
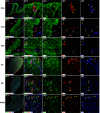
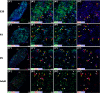
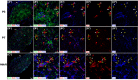
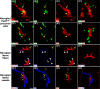
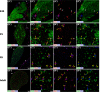
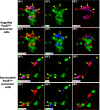
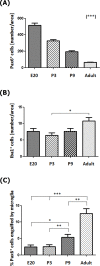
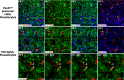
The adult pineal gland is composed of pinealocytes, astrocytes, microglia, and other interstitial cells that have been described in detail. However, factors that contribute to pineal development have not been fully elucidated, nor have pineal cell lineages been well characterized. We applied systematic double, triple and quadruple labeling of cell-specific markers on prenatal, postnatal and mature rat pineal gland tissue combined with confocal microscopy to provide a comprehensive view of the cellular dynamics and cell lineages that contribute to pineal gland development. The pineal gland begins as an evagination of neuroepithelium in the roof of the third ventricle. The pineal primordium initially consists of radially aligned Pax6+ precursor cells that express vimentin and divide at the ventricular lumen. After the tubular neuroepithelium fuses, the distribution of Pax6+ cells transitions to include rosette-like structures and later, dispersed cells. In the developing gland all dividing cells express Pax6, indicating that Pax6+ precursor cells generate pinealocytes and some interstitial cells. The density of Pax6+ cells decreases across pineal development as a result of cellular differentiation and microglial phagocytosis, but Pax6+ cells remain in the adult gland as a distinct population. Microglial colonization begins after pineal recess formation. Microglial phagocytosis of Pax6+ cells is not common at early stages but increases as microglia colonize the gland. In the postnatal gland microglia affiliate with Tuj1+ nerve fibers, IB4+ blood vessels, and Pax6+ cells. We demonstrate that microglia engulf Pax6+ cells, nerve fibers, and blood vessel-related elements, but not pinealocytes. We conclude that microglia play a role in pineal gland formation and homeostasis by regulating the precursor cell population, remodeling blood vessels and pruning sympathetic nerve fibers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

