Differentiation of Glioblastoma from Brain Metastasis: Qualitative and Quantitative Analysis Using Arterial Spin Labeling MR Imaging
- PMID: 27861605
- PMCID: PMC5115760
- DOI: 10.1371/journal.pone.0166662
Differentiation of Glioblastoma from Brain Metastasis: Qualitative and Quantitative Analysis Using Arterial Spin Labeling MR Imaging
Abstract
Purpose: To evaluate the diagnostic performance of cerebral blood flow (CBF) by using arterial spin labeling (ASL) perfusion magnetic resonance (MR) imaging to differentiate glioblastoma (GBM) from brain metastasis.
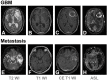
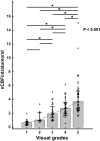
Materials and methods: The institutional review board of our hospital approved this retrospective study. The study population consisted of 128 consecutive patients who underwent surgical resection and were diagnosed as either GBM (n = 89) or brain metastasis (n = 39). All participants underwent preoperative MR imaging including ASL. For qualitative analysis, the tumors were visually graded into five categories based on ASL-CBF maps by two blinded reviewers. For quantitative analysis, the reviewers drew regions of interest (ROIs) on ASL-CBF maps upon the most hyperperfused portion within the tumor and upon peritumoral T2 hyperintensity area. Signal intensities of intratumoral and peritumoral ROIs for each subject were normalized by dividing the values by those of contralateral normal gray matter (nCBFintratumoral and nCBFperitumoral, respectively). Visual grading scales and quantitative parameters between GBM and brain metastasis were compared. In addition, the area under the receiver-operating characteristic curve was used to evaluate the diagnostic performance of ASL-driven CBF to differentiate GBM from brain metastasis.
Results: For qualitative analysis, GBM group showed significantly higher grade compared to metastasis group (p = 0.001). For quantitative analysis, both nCBFintratumoral and nCBFperitumoral in GBM were significantly higher than those in metastasis (both p < 0.001). The areas under the curve were 0.677, 0.714, and 0.835 for visual grading, nCBFintratumoral, and nCBFperitumoral, respectively (all p < 0.001).
Conclusion: ASL perfusion MR imaging can aid in the differentiation of GBM from brain metastasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Differentiation between primary CNS lymphoma and glioblastoma: qualitative and quantitative analysis using arterial spin labeling MR imaging.Eur Radiol. 2018 Sep;28(9):3801-3810. doi: 10.1007/s00330-018-5359-5. Epub 2018 Apr 4. Eur Radiol. 2018. PMID: 29619520
-
Differences In High-Intensity Signal Volume Between Arterial Spin Labeling And Contrast-Enhanced T1-Weighted Imaging May Be Useful For Differentiating Glioblastoma From Brain Metastasis.J Med Invest. 2017;64(1.2):58-63. doi: 10.2152/jmi.64.58. J Med Invest. 2017. PMID: 28373629
-
The role of cerebral blood flow gradient in peritumoral edema for differentiation of glioblastomas from solitary metastatic lesions.Oncotarget. 2016 Oct 18;7(42):69051-69059. doi: 10.18632/oncotarget.12053. Oncotarget. 2016. PMID: 27655705 Free PMC article.
-
Arterial Spin Labeling and Amide Proton Transfer Imaging can Differentiate Glioblastoma from Brain Metastasis: A Systematic Review and Meta-Analysis.World Neurosurg. 2024 Feb;182:e702-e711. doi: 10.1016/j.wneu.2023.12.023. Epub 2023 Dec 9. World Neurosurg. 2024. PMID: 38072160
-
Machine learning applications for the differentiation of primary central nervous system lymphoma from glioblastoma on imaging: a systematic review and meta-analysis.Neurosurg Focus. 2018 Nov 1;45(5):E5. doi: 10.3171/2018.8.FOCUS18325. Neurosurg Focus. 2018. PMID: 30453459
Cited by
-
Based on arterial spin labeling helps to differentiate high-grade gliomas from brain solitary metastasis: A systematic review and meta-analysis.Medicine (Baltimore). 2019 May;98(19):e15580. doi: 10.1097/MD.0000000000015580. Medicine (Baltimore). 2019. PMID: 31083237 Free PMC article.
-
A glutamatergic biomarker panel enables differentiating Grade 4 gliomas/astrocytomas from brain metastases.Front Oncol. 2024 May 21;14:1335401. doi: 10.3389/fonc.2024.1335401. eCollection 2024. Front Oncol. 2024. PMID: 38835368 Free PMC article.
-
Brain metastasis.Nat Rev Cancer. 2020 Jan;20(1):4-11. doi: 10.1038/s41568-019-0220-y. Epub 2019 Nov 28. Nat Rev Cancer. 2020. PMID: 31780784 Review.
-
Perfusion measurement in brain gliomas using velocity-selective arterial spin labeling: comparison with pseudo-continuous arterial spin labeling and dynamic susceptibility contrast MRI.Eur Radiol. 2022 May;32(5):2976-2987. doi: 10.1007/s00330-021-08406-7. Epub 2022 Jan 23. Eur Radiol. 2022. PMID: 35066634
-
Brain metastases: neuroimaging.Handb Clin Neurol. 2018;149:89-112. doi: 10.1016/B978-0-12-811161-1.00007-4. Handb Clin Neurol. 2018. PMID: 29307364 Free PMC article. Review.
References
-
- Cha S, Lupo JM, Chen MH, Lamborn KR, McDermott MW, Berger MS, et al. (2007) Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 28: 1078–1084. 10.3174/ajnr.A0484 - DOI - PMC - PubMed
-
- Kelly PJ, Daumas-Duport C, Scheithauer BW, Kall BA, Kispert DB (1987) Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc 62: 450–459. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

