The Systematic Investigation of the Quorum Sensing System of the Biocontrol Strain Pseudomonas chlororaphis subsp. aurantiaca PB-St2 Unveils aurI to Be a Biosynthetic Origin for 3-Oxo-Homoserine Lactones
- PMID: 27861617
- PMCID: PMC5115851
- DOI: 10.1371/journal.pone.0167002
The Systematic Investigation of the Quorum Sensing System of the Biocontrol Strain Pseudomonas chlororaphis subsp. aurantiaca PB-St2 Unveils aurI to Be a Biosynthetic Origin for 3-Oxo-Homoserine Lactones
Abstract
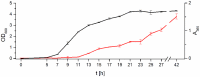
The shoot endophytic biocontrol strain Pseudomonas chlororaphis subsp. aurantiaca PB-St2 produces a wide range of exoproducts, including enzymes and antibiotics. The production of exoproducts is commonly tightly regulated. In order to get a deeper insight into the regulatory network of PB-St2, the strain was systematically investigated regarding its quorum sensing systems, both on the genetic and metabolic level. The genome analysis of PB-St2 revealed the presence of four putative acyl homoserine lactone (AHL) biosynthesis genes: phzI, csaI, aurI, and hdtS. LC-MS/MS analyses of the crude supernatant extracts demonstrated that PB-St2 produces eight AHLs. In addition, the concentration of all AHL derivatives was quantified time-resolved in parallel over a period of 42 h during the growth of P. aurantiaca PB-St2, resulting in production curves, which showed differences regarding the maximum levels of the AHLs (14.6 nM- 1.75 μM) and the production period. Cloning and heterologous overexpression of all identified AHL synthase genes in Escherichia coli proved the functionality of the resulting synthases PhzI, CsaI, and AurI. A clear AHL production pattern was assigned to each of these three AHL synthases, while the HdtS synthase did not lead to any AHL production. Furthermore, the heterologous expression study demonstrated unequivocally and for the first time that AurI directs the synthesis of two 3-oxo-AHLs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Complete Genome Sequence of Pseudomonas chlororaphis subsp. aurantiaca Reveals a Triplicate Quorum-Sensing Mechanism for Regulation of Phenazine Production.Microbes Environ. 2017 Mar 31;32(1):47-53. doi: 10.1264/jsme2.ME16162. Epub 2017 Feb 25. Microbes Environ. 2017. PMID: 28239068 Free PMC article.
-
Phenazine antibiotic production and antifungal activity are regulated by multiple quorum-sensing systems in Pseudomonas chlororaphis subsp. aurantiaca StFRB508.J Biosci Bioeng. 2013 Nov;116(5):580-4. doi: 10.1016/j.jbiosc.2013.04.022. Epub 2013 May 30. J Biosci Bioeng. 2013. PMID: 23727350
-
Identification, synthesis and regulatory function of the N-acylated homoserine lactone signals produced by Pseudomonas chlororaphis HT66.Microb Cell Fact. 2018 Jan 22;17(1):9. doi: 10.1186/s12934-017-0854-y. Microb Cell Fact. 2018. PMID: 29357848 Free PMC article.
-
An evolving perspective on the Pseudomonas aeruginosa orphan quorum sensing regulator QscR.Front Cell Infect Microbiol. 2014 Oct 28;4:152. doi: 10.3389/fcimb.2014.00152. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25389523 Free PMC article. Review.
-
Insights into plant-beneficial traits of probiotic Pseudomonas chlororaphis isolates.J Med Microbiol. 2020 Mar;69(3):361-371. doi: 10.1099/jmm.0.001157. J Med Microbiol. 2020. PMID: 32043956 Review.
Cited by
-
Investigation of the quorum-sensing regulon of the biocontrol bacterium Pseudomonas chlororaphis strain PA23.PLoS One. 2020 Feb 28;15(2):e0226232. doi: 10.1371/journal.pone.0226232. eCollection 2020. PLoS One. 2020. PMID: 32109244 Free PMC article.
-
Evaluation of Soil Streptomyces spp. for the Biological Control of Fusarium Wilt Disease and Growth Promotion in Tomato and Banana.Plant Pathol J. 2023 Feb;39(1):108-122. doi: 10.5423/PPJ.OA.08.2022.0124. Epub 2023 Feb 1. Plant Pathol J. 2023. PMID: 36760053 Free PMC article.
-
Recent Developments in the Biological Activities, Bioproduction, and Applications of Pseudomonas spp. Phenazines.Molecules. 2023 Feb 1;28(3):1368. doi: 10.3390/molecules28031368. Molecules. 2023. PMID: 36771036 Free PMC article. Review.
-
Bacterial Lighthouses-Real-Time Detection of Yersinia enterocolitica by Quorum Sensing.Biosensors (Basel). 2021 Dec 16;11(12):517. doi: 10.3390/bios11120517. Biosensors (Basel). 2021. PMID: 34940274 Free PMC article.
-
Draft Genome Sequence of Lipopeptide-Producing Strain Pseudomonas fluorescens DSM 11579 and Comparative Genomics with Pseudomonas sp. Strain SH-C52, a Closely Related Lipopeptide-Producing Strain.Microbiol Resour Announc. 2020 May 21;9(21):e00304-20. doi: 10.1128/MRA.00304-20. Microbiol Resour Announc. 2020. PMID: 32439665 Free PMC article.
References
-
- Singh K, Singh RP. Red rot In: Ricaud C, Egan BT, Gillaspie AGJ, Hughes CG, editors. Diseases of sugarcane: major diseases: Elsevier Science, New York; 2012.
-
- Kibria G, Yousuf Haroon AK, Nugegoda D, Rose G. Climate change and chemicals Environmental and biological aspects. Pitam Pura, New Delhi: New India Publishing Agency; 2010.
-
- Kookana RS, Baskaran S, Naidu R. Pesticide fate and behaviour in Australian soils in relation to contamination and management of soil and water: a review. Soil Research. 1998;36(5): 715–764.
-
- Wightwick A, Allinson G. Pesticide residues in Victorian waterways: a review. Australas J Ecotoxicol. 2007;13(3): 91–112.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

